As described in this article, the CSDA10F dual-arm robot (Yaskawa, Kitakyushu, Japan) can utilize pipets, syringes, vials, microplates and thermal shakers (including pipets and a thermal shaker designed for manual use) to prepare and transfer samples.1 When sample preparation is complete, samples are transferred to a GC/MS (gas chromatography–mass spectrometry) or LC/MS (liquid chromatography–mass spectrometry) autosampler (see Figure 1).1–3
 Figure 1 – CSDA10F dual-arm robot (1) picking up a pipet, (2) LC/MS, (3) GC/MS, (4) pipets on shelf and (5) deck for labware.
Figure 1 – CSDA10F dual-arm robot (1) picking up a pipet, (2) LC/MS, (3) GC/MS, (4) pipets on shelf and (5) deck for labware.Programming and operation
The robot must be programmed to handle different labware and devices for full automation to be achieved. A series of robot programs, or robot jobs, were created to eliminate the need to reprogram the robot to handle different samples.2 These programs make up the motion database. Several robot jobs are selected from the motion database and are then combined to carry out a task (see below).
For liquid handling, the dual-arm robot uses manual pipets and labware such as glass vials, microplates and reservoirs. The robot uses a simple microplate shaker, a thermal shaker for single vials or microplates and an ultrasonic bath for homogenizing solutions. Operations include sample transfer, opening/closing doors and lids, pressing a button or turning a knob to start/stop.
System integration
SAMI Workstation EX (Beckman Coulter, Brea, Calif.) process control software provides a GUI (graphical user interface) that enables easy process or assay design4 and process scheduling functionality even by those without robotic programming knowledge. The software incorporates optimized planning and data-driven dynamic rescheduling for prevalidated schedules and run-time flexibility. Figure 2 shows a SAMI program for opening vials. Labware positions, processes and devices are represented by icons connected by lines; the arrow indicates flow direction. When the SAMI program is executed, the robot selects the vials and removes and places their lids at the chosen positions. Users can design processes for pipetting, shaking and transferring labware or samples. A software interface written in C# integrates the robot into the software system (see Figure 3 caption for details).
 Figure 2 – SAMI program for opening vials. Left: icon is used to select vials with different volumes and positions on the rack. “Remove lid” represents process to remove the lid from the labware. In the flowing process, the Labware slot is connected to a Home icon, meaning that the vial will be placed back in the home position from which it was chosen. The Lid slot is connected to the Change Home icon, which is used to redefine new Home positions for lids, and is then connected to another Home icon. Thus the lids will be released to new defined positions.
Figure 2 – SAMI program for opening vials. Left: icon is used to select vials with different volumes and positions on the rack. “Remove lid” represents process to remove the lid from the labware. In the flowing process, the Labware slot is connected to a Home icon, meaning that the vial will be placed back in the home position from which it was chosen. The Lid slot is connected to the Change Home icon, which is used to redefine new Home positions for lids, and is then connected to another Home icon. Thus the lids will be released to new defined positions.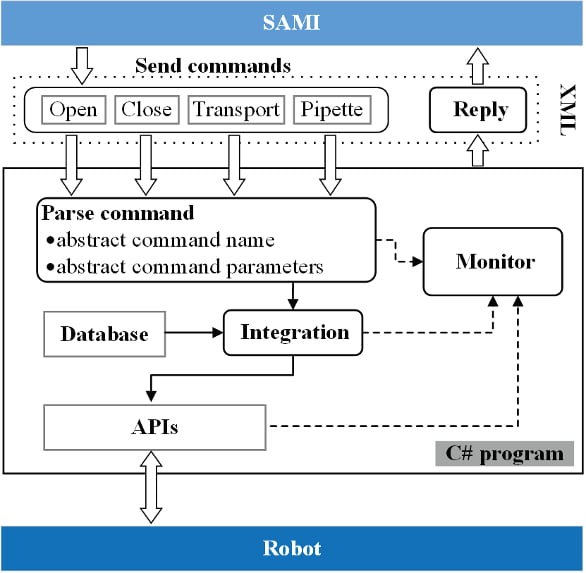 Figure 3 – Integration of the robotic system with SAMI software. 1) The C# program interacts with the software by sending/receiving data in XML format. When a SAMI program is executed, it sends commands to the C# program, which can be used to command the robot to open/close labware, transport labware or pipet liquids. Once a command is received, the C# program parses it to abstract its name and parameters. The name and parameters of the commands are used to select related robot jobs from the database, which will be then integrated. 2) The C# program is integrated with the robot based on Yaskawa MOTOCOM32 software. Through the software’s application programming interfaces, the C# program can monitor the status of the robot, transfer files to the robot and so on. When a command is executed successfully or an error occurs, the C# program relays the message to the SAMI program
Figure 3 – Integration of the robotic system with SAMI software. 1) The C# program interacts with the software by sending/receiving data in XML format. When a SAMI program is executed, it sends commands to the C# program, which can be used to command the robot to open/close labware, transport labware or pipet liquids. Once a command is received, the C# program parses it to abstract its name and parameters. The name and parameters of the commands are used to select related robot jobs from the database, which will be then integrated. 2) The C# program is integrated with the robot based on Yaskawa MOTOCOM32 software. Through the software’s application programming interfaces, the C# program can monitor the status of the robot, transfer files to the robot and so on. When a command is executed successfully or an error occurs, the C# program relays the message to the SAMI programExperimental
The experiments below demonstrate performance of the CSDA10F dualarm robot.
Determining enantiomeric excess of chiral compounds
This process5,6 included preparation of standard and sample solutions, derivatization and final dilution. Following the sample assay process, designed by the user with SAMI software, the robot carried out sample preparation with the same laboratory equipment used in the manual procedure. The samples prepared on 96-well multiple-well plates were fed to the autosampler for injection into the mass spectrometer.
Determining enantiomeric excess of proline
Samples were prepared by the robot and then analyzed on an Agilent LC/ MS system (Agilent Technologies, Palo Alto, Calif.) comprising a G1379B vacuum degasser, G1312B binary pump, G1367C high-performance automated liquid sampler and G1969A time-of-flight mass spectrometer (TOF-MS) with electrospray ionization (ESI). Data acquisition and processing were performed using MassHunter Data Acquisition and MassHunter Qualification software (Agilent Technologies). Figure 4 shows a mass spectrum with the characteristic masses m/z = 394.13 and 408.14 of the detected [M-H]– ions of the two derivatives of a sample. The ratio of the characteristic m/z values of the derivatives was used to determine enantiomeric excess.
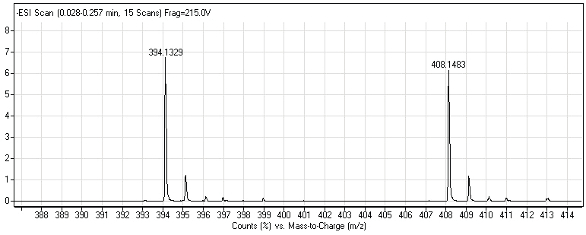 Figure 4 – Mass spectrum of [M-H]– ions of the derivatives of proline.
Figure 4 – Mass spectrum of [M-H]– ions of the derivatives of proline.Summary
The CSDA10F dual-arm robot effectively automates sample preparation for the life science laboratory. Operators can design processes with the user-friendly GUI provided by SAMI Workstation EX software, without having prior experience with robotic systems.
References
- Fleischer, H.; Drews, R.R. et al. JALA 2016; http://jla.sagepub.com/content/early/2016/03/19/2211068216637352.abstract
- Chu, X.; Fleischer, H. et al. IEEE I2MTC 2015, 500–4.
- Chu, X.; Fleischer, H. et al. IEEE CASE 2015, 979–84.
- www.beckmancoulter.com/wsrportal/WSR/research-and-discovery/index.htm
- Fleischer, H. and Thurow, K. Am. Lab. 2011, 43(9), 32–6.
- Fleischer, H. and Thurow, K. Amino Acids 2013, 44(3), 1039–51.
Additional reading
- South, S.F.; Casina, T.S., et al. Transfusion 2012, 52(8), 81S–87S.
- Drotning, W.; Wapman W. et al. ASCE 1996, 241–7.
- Puccinelli, J.P.; Su X. et al. JALA 2010, 15(1), 25–32.
- Kong, F.; Yuan, L. et al. JALA 2012, 17(3), 169–85.
- Bogue, R. Ind. Robot. 2012, 39(2), 113–9.
- Moore, K.W.; Newman, R. et al. JALA 2007, 12(2), 115–23.
- Smith, C.; Karayiannidis, Y. et al. Robot. Auton. Syst. 2012, 60(10), 1340– 53.
- Ott, C. and Eiberger, O. et al. IEEE RAS 2006, 276–83.
- Zanchettin, A.M.; Bascetta, L. et al. Appl. Ergon. 2013, 44(6), 982–9.
- Kock, S.; Vittor, T. et al. IEEE ISAM 2011, 1–5.
Xianghua Chu, Thomas Roddelkopf, Ph.D., and Kerstin Thurow, Ph.D., are with the Center for Life Science Automation (celisca), University of Rostock, Friedrich-Barnewitz Str. 8, D-18119 Rostock, Germany; tel.: +49 381 498 7803; fax: +49 381 498 7802; e-mail: [email protected]; www.celisca.de. Heidi Fleischer, Ph.D., and Norbert Stoll, Ph.D., are with the Institute of Automation (IAT), University of Rostock. Michael Klos, Ph.D., is with Yaskawa Europe GmbH, Allershausen, Germany. The authors wish to thank Lars Woinar, Heiko Engelhardt and Steffen Junginger (Institute of Automation [IAT], University of Rostock) for their technical support and suggestions.