Over the past 30 years, liquid chromatography has proven to be the predominant analytical technology within laboratory-dependent organizations around the world. No other single technology supports a company’s analytical needs from product discovery, research, all stages of development, scaling, and manufacturing to final testing for release with the performance and efficiency of LC.
Yet even with its role as a central analytical tool, not too long ago LC technology came dangerously close to becoming a scientific commodity where new systems and methods merely offered incremental advancements with a relatively minor competitive advantage. While the world of technology took great leaps forward, analytical chemistry lagged behind. High-performance liquid chromatography has not kept pace with the urgency of today’s business demands to analyze more samples, faster, and with better results.
Six years ago, a new category of chromatographic performance emerged with the commercialization of sub-2-µm particle columns engineered in tandem with advanced instrument design featuring modern fluidics modules that deliver very high performance. The result of smaller column material particle sizes and reduced system dispersion has been significant improvements in analytical resolution and sensitivity while increasing sample throughput.
Waters Corp. (Milford, MA) is widely recognized for fueling this LC revolution in 2004 with the introduction of Ultra- Performance Liquid Chromatography®, better known today as UPLC®. This disruptive technology, first released as the ACQUITY® UPLC system, has already replaced thousands of HPLC systems, supported more than 500 peer-reviewed papers, demonstrated reduced solvent consumption up to 95% for greener laboratories, and has served the needs of regulatory agencies around the globe.
System description
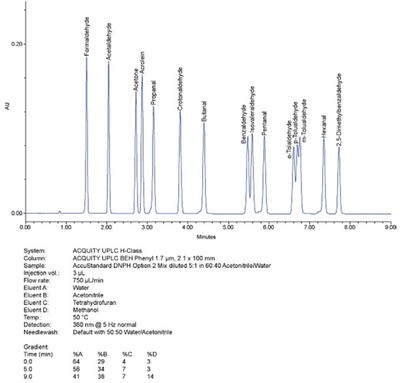
Figure 1 - The ability to form a quaternary gradient on the ACQUITY UPLC H-Class system improves the critical resolution for U.S. EPA Method 8315 Option 2 for the analysis of 15 aldehydes and ketones as 2,4-dinitrophenylhydrazine (DNPH) derivatives, with a UPLC separation in less than 10 min. (Figures courtesy of Mike Jones and Tanya Jenkins, Waters Corp.)
Having proven its performance and reliability under extremely rigorous conditions for the most demanding applications, UPLC technology has brought routine benefits to increasing numbers of scientists, so much so that today’s HPLC users, many of whom are not able to or prefer not to change their approach to LC, are looking for the benefits from UPLC technology in a way that is compatible with their laboratory procedures. With that goal in mind, the ACQUITY UPLC H-Class system was developed, which provides UPLC performance with HPLC familiarity.
Achieving UPLC-quality separations without changing how HPLC operators work was identified by Waters customers as the key challenge to accelerating their adoption of UPLC. The answer to this hurdle came in the form of the ACQUITY UPLC H-Class’s quaternary solvent manager (QSM) and sample manager with flow-through needle (SM-FTN) design (see Figure 1).
The ACQUITY UPLC, the original system, features a binary solvent manager (BSM) with fixed-loop sample manager. The BSM-based ACQUITY UPLC system is characterized by lower system volume (dwell volume), minimized dispersion, and fast injection-to-injection cycle times. The system is well-suited for high throughput and particularly any MS-based application where its low system dispersion provides high resolution and sensitivity. The system represents the lowest in solvent consumption and highest-efficiency performance for research-grade applications.
The ACQUITY UPLC H-Class system, with its quaternary solvent manager and sample manager, with flow-through needle design, features a nearly identical work flow compared with a traditional HPLC system. The system technology maintains the ACQUITY UPLC’s low dispersion characteristic, enabling the chromatographer to achieve the same high-efficiency separations as the original system. The QSM module offers expanded solvent blending capabilities, and the SM-FTN features an intuitive injector. The result is seamless upgrade of chromatographic capabilities.
In addition to simplifying the use of UPLC technology in HPLC applications, consistent separation chemistry is required throughout the transfer of methods. Method transfer kits from the manufacturer facilitate choosing an appropriate column to maintain the integrity of a separation when scaling from HPLC to UPLC and back. In fact, the method transfer process can be accomplished in three steps:
- Select an ACQUITY UPLC column that is comparable to the current HPLC column using the Waters reversed-phase column selectivity chart.
- Enter existing HPLC conditions into the ACQUITY UPLC columns calculator. The tool guides users step-by-step to either scale the gradient or further optimize for speed or resolution. The best column dimensions are selected from the menu, and the best method conditions are calculated.
- Run the analysis.
Figure 2 - In this method transfer example using the related substances test for galantamine, used in the treatment of Alzheimer’s disease, the USP method (monograph: USP32-NF27 supplement: no. 2, page 4245) is demonstrated first using an HPLC system. The method is directly transferred to the ACQUITY UPLC H-Class system using an HPLC column, maintaining selectivity and resolution, then scaled to UPLC using the ACQUITY UPLC columns calculator and optimized for the shortest analysis time at equal peak capacity. The run time decreased by 46 min.
The multisolvent blending capabilities of the ACQUITY UPLC H-Class system also make it an effective platform for methods development. A series of method development kits consist of several UPLC columns that encompass a range of selectivities to accommodate different method development approaches. The kits enable methods to be developed efficiently and effectively on the system.
Backed by a range of ACQUITY UPLC columns that includes three particle substrates in 11 chemistries, all of which are scaleable between HPLC and UPLC particle sizes, the ACQUITY UPLC H-Class system supports a broad range of application needs right from its introduction (see Figure 2). It makes the productivity and chromatographic performance of UPLC accessible to HPLC users who need the flexibility of a quaternary-based system.
Peptide mapping separation
Early ACQUITY UPLC H-Class evaluations indicate applicability across a broad range of routine analysis applications, including the food, environmental, petrochemical, and life science industries. As an example, an important bioseparation application will be examined. The most common chromatographic technique utilized for peptide mapping is reversed-phase HPLC (RP-HPLC), in which resolution is a primary criterion for success.
The gradients for RP-HPLC tend to be very shallow (<1% change in organic/min) and run times are typically hours. A binary system is usually selected based on the perception that its pump is better able to run shallow gradients with minimal delay volume and accurate mixing. Some typical solvent compositions are 0.1% TFA in water/acetonitrile or 0.1% formic acid in water/acetonitrile. Typically, columns are long (150 and 250 mm), are 2.1 mm in diameter, and contain 1.7 and 3 µm (130 Å or 300 Å) silica-based C8 or C18 particles. Usually these separations are run with flow rates of 0.1–0.4 mL/min.
Selectivity can be adjusted with the choice of mobile phase modifier, with the concentration of that modifier, and with the selection of the organic solvent. Evaluating the combinations of these parameters with a binary gradient system requires manual preparation of many combinations of solvents.
With the ACQUITY UPLC H-Class system, multisolvent blending eliminates manual solvent preparation. The solvent management system delivers either pure solvents or concentrated modifier solutions. The gradient proportioning valve then blends these components into the proportions that are required. This process is managed with AutoBlend technology, which automatically blends eluents in accurate proportions in any sequence of combinations. Programming different proportions from each line can generate a full range of solvent selectivities that are useful in peptide mapping, allowing separation development experiments to be executed in fully automated, unattended runs. This efficient method development approach reduces the risk of undetected modification to the protein.
This convenience in separation development is only useful if the system is robust and reliable. Chromatographic reproducibility is required for quantitative peptide maps; UPLC has been shown to provide good reproducibility while preserving peak shape and resolution of trace components. The ability of UPLC to improve peptide mapping separations has been demonstrated in the literature; it delivers greater resolution in combination with improved sensitivity compared to HPLC.
The ACQUITY UPLC H-Class has been evaluated for reproducibility with gradients typical of peptide mapping. The system has been used in both a binary gradient mode with premixed solvents and in a quaternary mode with on-line multisolvent blending. The results have been evaluated for consistency of retention time, preservation of peak shape and resolution, and reproducibility of quantitation. The binary and multisolvent preparations gave identical results. The reproducibility of retention time and resolution ensure unequivocal identification of each peak in the mixture with reliable relative quantitation.

Figure 3 - Solvent composition is easily adjusted using the AutoBlend capability of the ACQUITY UPLC H-Class system. In this example, the optimum concentration of TFA for a peptide map is identified simply by varying the percentage of flow taken from the D line. There is no need to make extra bottles of solvent, and intermediate values can be tested with minimal effort.
Temperature can be used for additional selectivity alteration for method development. Once a temperature is chosen for the execution of a peptide map, it is critical that the temperature is consistent. The system’s column heater, with an active inlet preheater, provides exacting control of the temperature in the column. This makes any peptide mapping run on the system more accurate, predictable, and transferable.
Finally, the flow-through needle design in the ACQUITY UPLC H-Class sample manager, in which the entire gradient flow specified for the analytical method flushes directly through the needle, reduces the risk of changing the composition of the sample or of losing sample as compared to systems that use the needle as a transfer device (see Figure 3).
ACQUITY UPLC family
The flagship ACQUITY UPLC platform no longer describes a single system. In addition to the ACQUITY UPLC system and the ACQUITY UPLC H-Class, the growing family of UPLC systems also includes the nanoACQUITY UPLC system and PATROL UPLC process analyzer.
The nanoACQUITY UPLC is designed for nanoscale, capillary, and narrow-bore separations to attain the highest chromatographic resolution, sensitivity, and reproducibility, especially for sample-limited analysis. The PATROL UPLC process analyzer moves UPLC analysis from off-line quality control laboratories directly to the manufacturing process, resulting in significant improvements in production efficiency.
The technology strategy is clearly focused on accelerating industry adoption of UPLC technology through a diverse range of systems based on a common, proven platform. Benefits of this approach reach well beyond targeted or individual laboratories. They are at the heart of today’s business, academic, and regulatory agency needs, ensuring that scientific, operational, sustainability, and profitability goals are met.
Mr. Foley is Director, LC Product Marketing; Ms. Gildea is Marketing Manager, Biopharmaceutical Business Operations; and Mr. Wheat is Principal Scientist, Systems Laboratory, Waters Corp., 34 Maple St., Milford, MA 01757, U.S.A.; tel.: 508-482-2000; e-mail: [email protected].