Ultrahigh-performance liquid chromatography (UHPLC) is a separation technique that utilizes columns packed with microparticles (<2 μm in diameter), as provided by the Xtreme liquid chromatography (X-LC™) system (JASCO Corp., Tokyo, Japan). UHPLC is gaining popularity due to its higher separation efficiency and low consumption of solvent. UHPLC separation times can be up to 10 times shorter than those required by conventional HPLC. However, the hardware for UHPLC must be designed carefully to minimize the peak dispersion of very narrow peaks generated by the technique.
Several extraction methods are used for a solid sample, such as classical solvent extraction and Soxhlet extraction.1,2 However, they require cumbersome pretreatment procedures and a lengthy extraction time, resulting in operating errors. Supercritical fluid extraction (SFE) has been investigated as an extraction method because it offers fast, cost-effective, and environmentally friendly extraction. These qualities are a result of the faster mass transfer in the supercritical fluid than in a liquid extractant. Carbon dioxide, which is used as the extraction medium in SFE, features mild critical parameters of Tc = 31.1 °C and Pc = 7.38 MPa, has low toxicity, and is inexpensive.
Hyphenating the multiple methods in an analysis system has many advantages, such as decreasing the analysis time, reducing the cost, simplifying sample pretreatment, and increasing the sensitivity and selectivity. In 1985, Sugiyama and co-workers reported on directly coupled SFE and supercritical fluid chromatography (SFC), and showed hyphenated analysis of caffeine from coffee beans.3 In 1987, Hawthorne and Miller described hyphenation of SFE-GC and analyzed polycyclic hydrocarbons and polychlorinated biphenyls from environmental solids.4 In 2003, Ashraf-Khorassani and co-workers investigated on-line SFC-LC for determining polymer additives.5 However, hyphenating SFE and UHPLC for the analysis of solid samples has yet to be reported.
The on-line SFE-UHPLC system (JASCO) employs valve-switching techniques. Components of interest in a solid sample are extracted effectively in the SFE system, automatically introduced into the UHPLC system, and subjected to rapid separation and detection with high sensitivity. The system offers very high speed and extremely sensitive analysis without complicated pretreatment.
Pepper is widely used as a spice. Piperine, the main constituent of pepper, is an alkaloid responsible for the sharp, pungent taste. In 1988, Takagi and co-workers studied the extraction efficiency of SFE of piperine from whole peppercorns treated by gamma ray.6 Piperine reportedly possesses several pharmacological characteristics.7–10 Since it is generally accepted as the predominant pungency in pepper, a quick and simple method to analyze piperine was needed.
Experimental
Apparatus
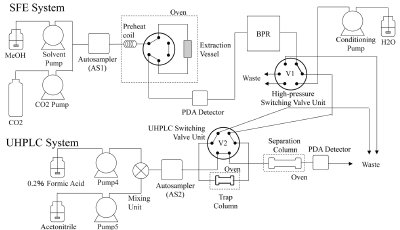
Figure 1 - Diagram of on-line SFE-UHPLC system.
The SFE-UHPLC hyphenated system was constructed from an SFE system and a UHPLC system (both from JASCO). Figure 1 shows the flow diagram of the on-line SFE-UHPLC system. Piperine solution was injected with an autosampler (AS1), or a pepper powder sample was placed in the extraction vessel. Target compounds in the sample were extracted under specified SFE conditions, and then trapped and concentrated on the trap column, which was connected in the high-pressure switching valve unit section. After the extraction, a conditioning solvent was delivered from the conditioning pump to remove the gaseous CO2 in the trap column. The trap column was then connected to the separation column by switching the UHPLC switching valve (V2), and the trapped compounds were separated by the separation column in the UHPLC system.
Chemicals
Carbon dioxide (99.99%) was purchased from Nippon Tansan, Co., Ltd. (Tokyo, Japan). Acetonitrile and methanol were from Wako Pure Chemicals (Osaka, Japan) and were used as modifiers (HPLC grade). Piperine (Wako Pure Chemicals) in methanol was prepared as a standard solution, with concentrations of 0.1 mg/mL. Ultrapure water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA). Samples were finely ground black and white pepper powders. Finely ground black and white pepper powders from S&B Foods (Tokyo, Japan) were obtained from a grocery store.
Preparation of extraction samples
The pepper samples for SFE were prepared using the following procedure: 1) Pepper powder was mixed with calcium sulfate dihydrate (1:999) (to dilute the pepper powder concentration in the sample to be extracted), 2) 10 mg of the mixed sample was placed in the extraction vessel, 3) the stainless steel rod was inserted to fill the vessel and fix the sample firmly to the bottom, and 4) the vessel cap was closed.
Results and discussion
Effect of temperature and pressure on SFE
The extraction efficiency varied with temperature and pressure.11 The effect of these parameters on the extraction efficiency was examined by changing the temperature of the extraction to 40 and 60 °C, and by changing the pressure to 10, 15, 20, 25, and 30 MPa. The results were very similar under all conditions. As the temperature of supercritical carbon dioxide becomes elevated at a constant pressure, the density and solvating power decrease. As the pressure becomes elevated at a constant temperature, the density of supercritical carbon dioxide increases and offers higher solvating power. Therefore, in this study, the extraction temperature was set to 40 °C and the pressure to 30 MPa.
Difference in trap column performance
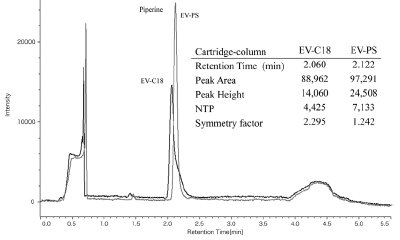
Figure 2 - Comparison of piperine chromatograms using EV-C18 and EV-PS cartridge columns.
- Column packings. The change in efficiency for trapping piperine (0.1 mg/mL, 1.0-μL injection) onto the trap column was studied by using two different types of trap column: an EV-C18 (ODS C18) cartridge column, and an EV-PS (polystyrene divinylbenzene copolymer) cartridge column. (Both cartridge columns are from JASCO.) Figure 2 compares the chromatograms of piperine obtained with these two columns and the peak parameters for piperine. It can be seen that the number of theoretical plates (NTP) and the symmetry factor for the EV-PS cartridge column are better than those for the EV-C18. Extracts were retained by hydrophobic interaction on C18 alkyl chains on the EV-C18 packing material; on the EV-PS packing material, extracts were retained on phenyl groups by π–π interaction between piperine and the packing material. The EV-PS loading capacity is larger than that of the EV-C18 because active sites are in the material of the EV-PS itself, whereas the active sites of EV-C18 are chemically bonded to the surface of porous silica gel particles. Therefore, the EV-PS column was used for the following piperine analysis.
Table 1 - Comparison of peak parameters for piperine
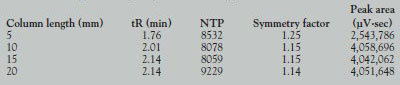
- Trap column length. The trapping efficiency was examined by changing the length of the trap column. Table 1 shows the peak parameters of piperine obtained by changing the length of the trap column. In the case of the 5-mm-long trap column, the NTP and the symmetry factor were better than those of the other length columns; however, the peak area was smaller than the other columns. This demonstrates that the 5-mm trap column cannot sufficiently trap the eluting compounds. Peak areas were the same for the 10-mm and longer columns. Since the NTP and the symmetry factor of the 20-mm-long column were the best among these columns, the authors decided to use the 20-mm trap column.
- Peak area and retention time reproducibilities. Peak area and retention time reproducibilities were calculated by five consecutive injections. The relative standard deviations for the retention times and peak areas were 0.36% and 1.91% (n = 5), respectively.
- Recovery. Recovery of piperine was calculated by the peak areas measured by the UHPLC system and the on-line SFE-UHPLC system. The recovery was 96.0%.
- Optimization of fractionation time. The SFE profile of the extracts from black pepper powder was monitored with an X-LC 3110MD photodiode array (PDA) detector (JASCO) to optimize the fractionation time. Extraction from pepper was completed in 0.3 min, which is less than classical solvent extraction methods. Fractionation was carried out from 0.0 to 0.5 min. The spectrum of the pepper extract was compared with a piperine spectrum. The correlation coefficient of the two spectra was 0.8265, showing that the extracts from the black pepper contained piperine.

Figure 3 - Sample: black pepper; a) SFE profiles at 345, 390, and 370 nm; aʹ) SFE profile contour plot; b) UHPLC chromatograms at 345, 390, and 370 nm; bʹ) UHPLC contour plot; and c) comparison of on-peak spectra of piperine standard and the peak in black pepper.
- Analysis of piperine in black and white pepper powder. Figure 3 shows the SFE extraction profile and UHPLC chromatograms of black pepper powder. The piperine peak in the black pepper powder was identified by retention time. Figure 3 also depicts the spectra of piperine standard and the piperine peak in the black pepper extract. The two spectra were perfectly matched, and the correlation coefficient was 1.0000. This demonstrates that piperine in the black pepper powder was extracted efficiently and separated cleanly by UHPLC in a short period of time. The content of piperine in the black pepper was found to be 55.6 mg/g by calculating the peak areas from the chromatograms of the piperine standard and black pepper. The reproducibility for the black pepper analysis was 4.53% (n = 5). White pepper powder was also analyzed using a similar method. The content of piperine in the white pepper was determined to be 34.6 mg/g. The reproducibility for the white pepper analysis was 3.73% (n = 5).
Table 2 - Procedures for the three extraction methods
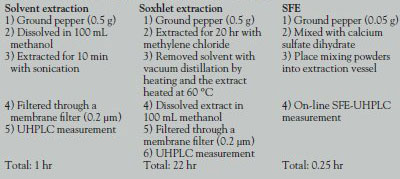
- Comparison of extraction method efficiency. In order to compare method efficiency, three different techniques were performed: simple solvent extraction, Soxhlet extraction, and on-line SFE-UHPLC (see Table 2). The procedure for the on-line SFE-UHPLC method is simple and pretreatment time is very short.
- Comparison of piperine recoveries.Table 3 shows the recoveries of piperine extraction in black and white pepper using the three extraction methods. The amount of piperine obtained by all three methods was about the same. This shows that the extraction of piperine by SFE offers comparable recoveries.
Table 3 - Contents and recoveries of piperine in black pepper and white pepper by the three extraction methods

Conclusion
Using UHPLC, it takes 1.7 min to analyze piperine, which is four times faster than HPLC (7.7 min). The X-LC UHPLC system and the SFE system were hyphenated successfully employing the column-switching techniques. Extracts containing piperine were trapped in the SFE system using an EV-PS cartridge column packed with styrene divinylbenzene copolymer. Good reproducibility in the UHPLC system was obtained, i.e., RSD%: 0.36% (retention time) and 1.91% (peak area). Overall recoveries were 96.0%.
The piperine spectra for both the standard sample and the pepper extract, measured with the X-LC 3110MD PDA detector, matched very well. Piperine in the black pepper was 56.6 mg/g; in white pepper it was 34.6 mg. The amount of piperine obtained by the three extraction methods was compared and was in good agreement. The on-line SFE-UHPLC method is very simple and offers high-speed analysis. The method will be useful for analyzing compounds in solid samples without complicated pretreatment or sample transfer.
References
- Hawthorne, S.B. Anal. Chem.1980, 62, 271.
- King, J.W.; France, J.E. In Analysis with Supercritical Fluids: Extraction and Chromatography, Wenclawiak, B., Ed. Springer: Berlin, 1992; p 32.
- Sugiyama, K.; Saito, M.; Honda, T.; Senda, M. J. Chromatogr.1985, 332, 107.
- Hawthorne, S.B.; Miller, D.J. J. Chromatogr.1987, 403, 63.
- Ashraf-Khorassani, M.; Nazem, N.; Taylor, L.T. J. Chromatogr. A 2003, 995, 227.
- Takagi, K.; Okuyama, T.; Yamauchi, Y.; Saito, M. Shokuhin Shosha 1988, 23(2), 46.
- Bajad, S.; Bedi, K.L.; Singla, A.K.; Johri, R.K. Planta Med. 2001, 67, 176.
- Bajad, S.; Bedi, K.L.; Singla, A.K.; Johri, R.K. Planta Med. 2001, 67, 284.
- Zhixiu, L.; Hoult, J.R.S.; Bennett, D.C.; Raman, A. Planta Med. 1999, 65, 600.
- Capasso, R.; Izzo, A.A.; Borrelli, F.; Russo, A.; Sautebin, L.; Pinto, A.; Capasso, F.; Mascolo, N. Life Sciences2002, 71, 2311.
- Wang, Z.; Ashraf-Khorassani, M.; Taylor, L.T. J. Chromatogr. A 2004, 1033, 221.
Mr. Yamaguchi, Mr. Kamezawa, Mr. Iwaya, Ms. Sato, Mr. Miyaji, Mr. Bounoshita, and Dr. Saito are with JASCO Corp., 2967-5 Ishikawa-machi Hachiozi-shi Tokyo 192-8537, Japan; tel.: +81 42 646 4111; e-mail: [email protected]. Mr. Tognarelli is with JASCO Inc., Easton, MD, U.S.A. This paper was presented in part as 2775-1P at Pittcon® 2010, Orlando, FL, U.S.A.