The use of a hydrogen peroxide vapor (H2O2) atomizer in situ to decontaminate a cell culture CO2 incubator without the use of heat sterilization offers significant advantages in routine clinical and highly regulated research laboratories in which costly down time must be avoided. The combination of a seven-minute H2O2 vapor fog in the chamber, circulated by the incubator airflow blower, followed by exposure to narrow-bandwidth ultraviolet light, establishes a thorough antimicrobial impact on all incubator walls, shelves, reservoirs, air plenums, sensors, and other interior components without the time and expense of high heat cycles, leaving only small amounts of sterile water droplets as a residual. Because all interior components are designed to remain in the chamber for sterilization during the process, use of a separate autoclave is avoided and the incubator can be returned to service in less than three hours.

Figure 1 - Sterisonic GxP MCO-19AIC (UVH) cell culture CO2 incubator.
In 2009, SANYO Electric Co. Ltd. (Gunma, Japan) introduced the Sterisonic™ GxP MCO-19AIC (UVH) cell culture CO2 incubator with H2O2 vapor sterilization (see Figure 1). The incubator complements the company’s proactive in situ contamination control systems first marketed in 2001. In a layered and orchestrated approach to cell culture incubation predicated on good laboratory technique, the addition of H2O2 vapor to an extensive arsenal of existing contamination control techniques, both passive and active, confronts a wide range of laboratory conditions and culture applications (see Figure 2).
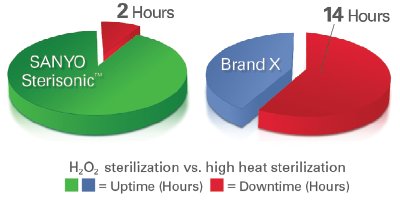
Figure 2 - The rapid in situ H2O2 sequence returns the fully sterilized Sterisonic GxP to normal use more quickly than other incubators.
The Sterisonic GxP cell culture solution offers the following benefits:
- Good laboratory technique
- Intelligent cabinet design
- InCuSaFe™ copper-enriched interior walls
- New single-beam, dual-array infrared CO2 sensor with passive sampling
- Patented SafeCell™ UV decontamination cycling, in vitro
- H2O2 vapor sterilization process, in vitro.

Figure 3 - SANYO cell culture solution.
The cell culture solution (Figure 3) is based on a series of mutually dependent concentric systems working together to offer the safest, most productive in vitro cell culture environment possible. In addition to H2O2 sterilization, SANYO applies the combination of structural and materials engineering, new infrared sensor technology, self-compensating narrow bandwidth ultraviolet light, multipurpose airflow, intelligent microprocessor control, and graphical monitoring into a dynamic cell culture system designed to reward good laboratory technique for the most critical and highly regulated applications.
Evolution of H2O2 sterilization
The emergence of H2O2 vapor as a practical sterilization method has been well documented by numerous private and public agencies, and is receiving more attention at the bench level due, in part, to safety and efficacy when compared to ethylene oxide (EtO).1,2 In a review of commonly accepted sterilization techniques at the USP Annual Scientific Meeting, 20083 (presentation on Sterilization and Sterility Assurance), H2O2 vapor was categorically added to conventional methods such as chemical, dry heat, filtration, radiation, and steam sterilization for consideration in selecting the best technique for the desired application. As a condensing vapor, H2O2 is present in multiple phases simultaneously, requiring validation protocols to be constructed within the context of a liquid and gas hybrid. While the efficacy of H2O2 vapor ensures sterilization, the wide variation in sterilization process parameters among different products and applications requires that validation protocols associated with the cell culture incubator be ascertained from product-specific research in context with known outcomes in vastly different sterilization procedures. Because a consensus standard for H2O2 remains to be established, the concepts for general sterilizing agents outlined in ANSI/AAMI/ISO 14937 can be adopted as an appropriate validation strategy, along with other EtO standards that may apply. Additionally, several companies have obtained 510(k) clearance for the use of H2O2 vapor as a terminal sterilization technique for medical devices. Therefore, current practices suggest that validation for H2O2 vapor sterilization can be compared to EtO.
Advantages in GMP and GLP applications
Table 1 - Sterisonic GxP cell culture incubator system

Systems and design of the Sterisonic GxP incubator support both clinical and nonclinical applications, starting with research and leading into development, manufacturing, and quality control. As laboratories work to maintain contemporary tools and technologies in advance of new demands for both commercial and clinical success, selection of the laboratory incubator must include consideration for scaleability and compliance. When retrofitting or building a new laboratory, laboratory planners must anticipate reporting and datalogging performance of laboratory incubators heretofore classified as commodity equipment, but now recognized as a critical link in the chain of custody for quality management and validation.4
The Sterisonic GxP incubator offers significant advantages in complying with GMP and GLP criteria imposed by outside and internal regulatory agencies or process manuals (Table 1). With respect to GMP, the incubator includes relational operating systems and safeguards designed to protect the cell culture or cell expressed product, particularly when associated with direct human application such as in vitro fertilization (IVF), stem cells, regenerative tissue processes, or autologous cell culture (Table 2). GLP criteria promoting continuity in technique and preserving the acquisition and integrity of performance data associated with the typical incubator performance as well as the sterilization cycle are accommodated through the integral control and monitoring system, complete with data point logging and archiving, and optional communications for remote or off-site monitoring. In developing the Sterisonic contamination control model, SANYO engineers based their H2O2 design on well-documented efficacy of the increasingly popular hydrogen peroxide vapor sterilization technique often used in decontamination of biological safety cabinets, environmental chambers, and other enclosures. When H2O2 vapor is deployed in association with the narrow- bandwidth ultraviolet light decontamination system already designed into the incubator, the complete sterilization process is safe, effective, and significantly faster than conventional high-heat decontamination solutions.
Table 2 - Typical applications for Sterisonic GxP

Sterisonic GxP contamination control system
The H2O2 incubator sterilization system in vitro is an extension of the SANYO Active Background Contamination Control™ technique introduced by SANYO Electric Biomedical Co., Ltd. in 2001. Now part of the MCO-19AIC (UVH) incubator series, the cell culture CO2 incubator employs an isolated narrow-bandwidth UV light to destroy airborne contaminants in the incubator chamber, as well as water-borne organisms in the humidity water reservoir. Integrated with copper-enriched interior surfaces and components that inhibit the growth of organisms without surface discoloration, the incubator offers an optimum cell culture environment that protects cultures in vitro, and minimizes frequent chamber cleaning and down time.
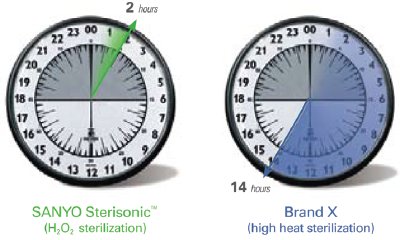
Figure 4 - H2O2 sterilization vs high-heat sterilization.
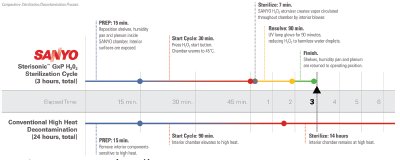
Figure 5 - Comparative sterilization/decontamination process.
In 2006, comparative testing commissioned by SANYO and performed by a certified independent testing laboratory confirmed that the SANYO UV light sterilization process is as effective against bacteria, yeasts, and molds as high heat sterilization at sustained temperatures ranging from 90 °C to 140 °C offered in competitive products (Figures 4 and 5). Additionally, the SANYO incubator isolates the UV emission from cell cultures during normal operation to permit sterilization of the internal atmosphere following routine door openings without damaging cell cultures, a process that cannot be replicated by a heat sterilization technique (Table 3).
Table 3 - H2O2 versus heat sterilization*

Productivity advantage
Automatically coordinated processes within the Sterisonic GxP cell culture incubator conditions of temperature, humidity, and CO2 control while arresting contamination. When complete sterilization is required, the Sterisonic H2O2 sequence offers an important uptime advantage using high heat or conventional decontamination. The 3-hr in situ sterilization sequence returns the incubator to service more quickly and with greater efficiency using high heat or other decontamination protocols. In applications that require frequent sterilization between processes, the incubator yields a significant advantage in productivity.
Maximum productivity
When complete sterilization is required, the Sterisonic H2O2 sequence offers a cost-effective, time-saving advantage using high heat or conventional determination.