Many problems in gas chromatography
follow a cylinder change-out, prompting
chemists to buy ever-higher purity grades
of gas. Yet, problems such as elevated
baseline noise, ghost peaks, and excessive
column bleed are often the result of contaminants
introduced by the gas management
system, not the cylinder. It can be
thought of as drinking clean water
through a dirty straw. This application
note discusses 10 steps to maintaining gas
stream purity, yielding more consistent
performance and operating savings.
Gas management systems
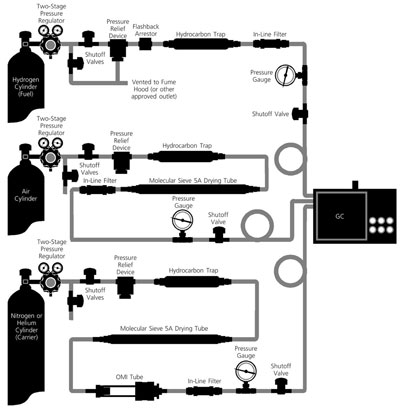
Figure 1 - Gas management system schematic. Reproduced with permission from Sigma-Aldrich
Co., Bulletin 898, T196898, 1996, p. 15.
Gas management systems contain many
components—gas source, regulators, tubing,
fittings, purifiers, traps, joints, and
valves—all of which can be a source of
leaks and contamination (see Figure 1). It
is relatively easy to contaminate a system
and time consuming to regain a baseline.
Contamination can contribute to column
damage and add gas and labor costs
from rerunning samples and longer
downtimes. The problem becomes especially
costly if users attempt to solve it by
buying a more expensive gas grade.
Instead, users can build a better gas management
system that will 1) contain and
remove contaminants before they do
damage, 2) provide a visual indication as
to when contaminants enter, and 3)
identify the contaminant. The following
10 steps are important to remember.
1. Choose the right gas grade. There is a
myth that higher-purity gas will
improve analytical results. If the
gas, no matter how pure, travels
through a dirty system, it becomes
contaminated. If the system is
clean, chromatographers may be
able to improve the baseline to
detect at lower levels or switch to a
lower purity level to save money.
To choose a purity level, the chromatographer
must first determine what
impurities can affect the process. The
gas supplier provides an analysis for
these contaminants, which should be
reviewed prior to purchase. The three
primary impurities that affect most GC systems are oxygen, moisture, and
hydrocarbons. Oxygen can accelerate
column bleed, reduce column life, and
change retention times. In some applications,
it can also cause ghosts or
unexpected peaks. Moisture can reduce
column life, shift retention times, and
increase baseline noise levels. Hydrocarbons
can also increase baseline
noise, degrade analyte quantification,
and cause ghost peaks. All three of
these contaminants are present in the
atmosphere; thus, preventing them
from mixing with the cylinder gas,
whatever the purity level, is critical.
2. Avoid contamination during cylinder
changes. Contamination most commonly
occurs when the connection
between the gas supply and the regulator
is broken. When the cylinder is
disconnected from the regulator, the
system goes to atmospheric pressure,
allowing atmosphere to enter. A properly
designed system with the right
components will minimize, contain,
and remove the contaminants. The
first line of defense is the connection
designated by the Compressed Gas
Association (CGA). This CGA nipple
should incorporate a high-purity,
nonlubricated check valve in its nose
to keep the system pressurized during
the cylinder change-out. Then, a block-and-bleed valve can contain
and remove the small amount of contaminants
that enter, while the downstream
system maintains pressure.
3. Use the right regulator. Another myth
is that any regulator with a stainless
steel diaphragm is suitable for analytical
service. That is only half
right. High-purity regulators use
stainless steel diaphragms since they
do not absorb contaminants. General-purpose regulators use neoprene
diaphragms, which can absorb and
then emit gas contaminants into the
gas stream unpredictably.
Body construction—bar stock or forged
body—is actually more important. A bar
stock regulator has a small internal volume
and a straight gas path. If contaminants
do enter, it is easy to predict when
they would emit. By contrast, a forged
body has a large internal cavity with no
direct path, allowing contaminants to
become trapped and emit unpredictably.
Only regulators made of bar stock,
cleaned for chromatographic service,
with a stainless steel diaphragm, should
be used for GC systems. Regulators
should never have lubricants or Teflon®
(DuPont, Wilmington, DE) tape on the
CGA nut, since these cause leaks and
contamination as well. The user should
consider whether a two-stage regulator is
needed to maintain a constant pressure
in the system. A single-stage regulator
will require frequent adjustment as cylinder
pressure decreases.

Figure 2 - Regulator mounting.
4. Mount cylinder regulators correctly.
Most regulators weigh approx. 8 lb
and are not easy to handle during a
cylinder change-out. If the weight is
unsupported, tubing can become
kinked, cracked, broken, or dislodged,
causing a leak. A simple remedy is to
use a wall-mounting bracket to support
the regulator (see Figure 2).
5. Use the proper tubing. Chromatographically
cleaned and “passivated”
stainless steel or copper tubing
should be used. Problems arise when
mixing metals. Brass regulator fittings,
for instance, will not achieve a
tight seal on stainless steel. Many
users purchase the correct tubing, but
contaminate it during installation.
For example, applying oil to the tubing
cutter may make it easier to use,
but may also introduce hydrocarbons
into the system. Cutters must be free
of lubricants, or the tubing must be
recleaned after cutting.

Figure 3 - Joint tightening. Reproduced with permission from Sigma-Aldrich Co.,
Bulletin 898, T196898, 1996, p. 11.
6. Make joints correctly. For compression
fittings, a two-piece ferrule made of
the same material as the tubing
should always be used. Brass ferrules
on stainless steel tubing,
for example, will not create a
tight seal. Another misconception
is that a loose fitting will
only allow gas to leak out of the
system. This is true when the
system is in a static state. However,
when gas is flowing, the
leak generates a vacuum that
can suck in atmosphere, which
contains oxygen, moisture, and
hydrocarbons. Conversely,
many people tend to overtighten
ferrules, which can crush
them, creating a leak that permits
these same contaminants to enter.
The ferrule should be tightened only
to the manufacturer’s specification
(see Figure 3). Also, if the system has
brazed joints, they must be made using
the fluxless brazing method. Orbital
welding is a viable alternative since it
creates a metal-to-metal fusion joint
that has no filler metal. Pipe thread
joints must only use Teflon tape without
a lubricant or sealer.
7. Use the right flexible pigtails. Flexible
pigtails are convenient, but those
with a Teflon core are porous
enough to allow small molecules of
helium and hydrogen to diffuse
through the wall. This can waste
more than 10% of the gas. Teflon
can also off-gas contaminants.
Instead, leak-tight, flexible pigtails
with internal stainless steel corrugated
bellows should be used.
8. Select the right valves. Valves are
another common contamination
source. Three common valves in
gas delivery systems are ball valves,
needle valves, and diaphragm packless
valves. Ball valves are popular
because they provide a visual indication
of whether they are opened
or closed. However, in helium and
hydrogen, these do not provide a
positive shut-off, and they require
lubrication, which may introduce contaminants to the system. Needle
valves provide flow control, but
may introduce contaminants from
lubricants and the potential offgassing
from the packing. The best
valves are diaphragm packless
valves, since they use multiple
metal diaphragms, no lubrication,
and are capable of passing helium
leak tests.
9. Install a cylinder changeover system.
Many chromatographers believe
that a cylinder change requires
4 hours to recover a baseline. This
recovery time is usually due to contaminants
entering the system.
Even with the safeguards described
above, a cylinder change-out can
take 30 min or more. Users too
often replace the cylinder when
convenient rather than when necessary.
They will change a cylinder
with more than 500 psig of pressure
remaining on a Friday afternoon to
avoid the expected downtime.

Figure 4 - Analytical changeover system.
In contrast, an automatic changeover
system allows for uninterrupted service,
eliminating waste and downtime.
When one cylinder is depleted,
the system switches to the other side,
allowing the empty to be returned
with a minimum of residual (150
psig). When selecting a changeover
system (see Figure 4), the chromatographer
should look for traits similar to
a regulator—bar stock construction,
nonlubricated, check valves, and
block-and-bleed valves.
10. Use purifiers only as a
backup. There are three
types of purifiers: surface
absorbent, chemically
absorbent, and
chemically absorbent
indicating. Purifiers
safeguard against contamination.
They are
not a practical method
for cleaning gas. In
fact, using some types
can actually introduce
impurities at unpredictable
moments.
Surface absorbent purifiers
are the worst choice.
When they become
40–60% saturated, the
internal velocity increases, and
impurities introduced during a
cylinder change-out can propel previously
captured contaminants into
the gas stream. When completely
saturated, the purifier may release
most of its contaminants at once,
requiring a long and arduous time to
clean the column.
Because chemically absorbent
purifiers lock up contaminants,
they cannot become dislodged
later. However, if the purifier is
nonindicating, it is difficult to
measure its saturation level. At
some point, the medium will stop
working, permitting contaminants
to pass through unabsorbed. Inline
purifiers also need valves on
both sides to prevent contaminants from entering the system
during a change-out.
An indicating purifier changes
color when it chemically absorbs
impurities. This color change gives
the user a visual indication that
impurities have entered and of
what type, and when to change the
purifier. In some indicating designs,
the purifier changes color from the
top down when impurities enter
from the gas source, and from the
bottom up when it detects contaminants
from downstream. A quick change
base with integral check
valves can also protect the gas
stream when changing a spent purifier
cartridge.
Conclusion
The right gas delivery system will
improve consistency, limit downtime,
and reduce costs. Chemists
should specify components and construction
methods to deliver clean
gas and preserve the integrity of their
systems. Ultimately, even the purest
gas is wasted if it passes through a
dirty straw.
Laboratories should seek suppliers
that understand chromatography
and will work with them to improve
their systems. For facilities requiring
larger amounts of gas, microbulk and
bulk supply options can ensure a
consistent supply of high-quality gas,
with fewer opportunities to introduce
contaminants. Use of these
larger modes also eliminates the
headaches associated with managing
a large number of cylinders. Ultimately,
maintaining gas stream
purity enables chemists to focus on
the work they do best.
Mr. Kandl is National Technical Manager, Specialty
Gas Equipment, Airgas, Inc., 259 N.
Radnor-Chester Rd., Ste. 100, Radnor, PA
19087-5253, U.S.A.; tel.: 800-255-2165; fax:
610-687-6932; e-mail: [email protected].