Out of a concern for purity and yield, many peptide
chemists today choose to extend their reaction times
excessively in order to obtain as high a purity and
yield as possible. Typical reaction times for conventional
Fmoc solid-phase peptide synthesis (SPPS) are
deprotection times of 10–30 min, and coupling times
of 20 min to over an hour, which can result in cycle
times of up to 2 hr. As a result, conventional peptide
synthesis chemistry is perceived as slow, but this is
not necessarily the case.
Historically, the biggest contribution to increasing
the speed of peptide synthesis was undoubtedly
the invention of solid-phase peptide synthesis
by Bruce Merrifield in 1963.1 By attaching
the growing peptide chain to a solid support,
his method eliminated time-consuming purification
steps and paved the way for automating the
process. Since then, advances in automation and
chemistry have made it possible to increase the
speed at which peptides can be made. Most chemistry
methods have focused on the development
of more efficient activators for the coupling step.
O-benzotriazole-N,N,N′,N′-tetramethyluronium
hexafluorophosphate (HBTU) was the first of
these activators, and was introduced in 1990
for performing FastMoc™ chemistry (Applied
Biosystems,
Foster City, CA) with coupling
times of 10–30 minutes.2
In 1993, Carpino introduced the activator O-(7-
azabenzotriazole-1-yl)-N,N,N′N′-tetramethyluronium
hexafluorophosphate (HATU), which was
used four years later by Alewood and Miranda to
perform 1–2 min couplings with Boc chemistry.3,4
Similar fast methods have not been developed for
Fmoc chemistry, and for many laboratories, HATU
is too expensive to use for all but the most difficult
couplings. In 2002, the activator 1H-benzotriazolium
1-[bis(dimethylamino)methylene]-5-chlorohexafluorophosphate
(1-),3-oxide (HCTU) was
introduced by Luxembourg Laboratories (Rehovat,
Israel), and is available at a significantly lower price
than HATU.5
The authors tested the efficiency of HCTU
by synthesizing a phosphorylated peptide
(H-CRRKGpSQKVS-NH2) using HBTU and
HCTU, and found that HCTU produced the higher-purity
peptide (data not shown). They then synthesized
the 65–74 fragment of the acyl carrier protein
(65–74ACP) (H-VQAAIDYING-OH) using HCTU;
HATU; HBTU; benzotriazol-1-yl-oxytripyrrolidinophosphonium
hexafluoro-phosphate (PyBOP);
and O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium
tetrafluoroborate (TBTU). They found that
HCTU and HATU produced peptides of extremely
similar purity, while the remaining activators had
additional impurities (data not shown). From this,
it was concluded that HCTU was a highly efficient
coupling reagent.
The goal was to synthesize peptides as quickly and
inexpensively as possible. The authors were able
to achieve extremely rapid coupling times using
the activator HCTU. This paper demonstrates
this on seven peptides with a variety of properties:
long, short, hydrophobic, hydrophilic, cyclic, and
peptides containing D-amino acids and pseudoproline
dipeptides.
Experimental
Peptides were synthesized as described previously
on either a Symphony® or Prelude™ peptide synthesizer
from Protein Technologies, Inc. (Tucson,
AZ).6 The Prelude automated peptide synthesizer
was used to save money on expensive monomer
additions, because its Single-Shot™ delivery feature
delivers the entire contents of an amino acid
bottle to a specified reaction vessel without priming
or waste. HPLC and mass spectrometry analysis
were also carried out as described previously.6
Results and discussion
Using HCTU as the coupling reagent, the
authors were able to synthesize the shorter peptides
(10 residues or less) using 1-min deprotection
times and 2-min coupling times. The
reaction times for the longer peptides (over 30
residues) were reduced to 2–3 min for deprotection
and 5 min for coupling, resulting in cycle
times of 14–19 min (Table 1). Specific results for
each peptide are detailed below.
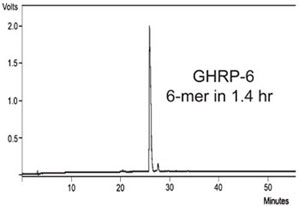
Figure 1 - HPLC of crude GHRP-6. This 6-mer peptide was synthesized
in 1.4 hr with 2 × 30 sec deprotection times and 2 × 1 min coupling times.
Peptide containing D-amino acids
GHRP-6 (H-HwAWfK-NH2) is a growth hormone
releasing peptide that contains two amino
acids with D-configuration (D-Trp and D-Phe).7
GHRP-6 was synthesized with 2 × 30 sec deprotection
times and 2 × 1 min coupling times,
resulting in a total synthesis time of 1.4 hr (Figure
1). D-amino acids were added using the Single-Shot delivery feature on the Prelude.

Figure 2 - HPLCs of crude a) linear and b) cyclized oxytocin. The
9-mer linear peptide was synthesized in 2.1 hr with 2 × 30 sec deprotection
times and 2 × 1 min coupling times. It was then cyclized for 2 × 40
min for a total synthesis time of 3.4 hr.
Oxytocin
Oxytocin (H-CYIQNCPLG-NH2) is a component
of the prohormone. It is located at the
N-terminal end of the sequence and contains a
disulfide bridge between Cys-1 and Cys-6. Linear
oxytocin was synthesized with deprotection
times of 2 × 30 sec and coupling times of 2 × 1
min, resulting in a total synthesis time of 2.1
hr (Figure 2a). It was cyclized on the resin for 2
× 40 min using thallium (III) trifluoroacetate
delivered by the Prelude’s Single-Shot delivery
feature prior to cleavage (Figure 2b). The cyclization
time was not optimized.
The 65–74 fragment of the acyl carrier protein
65–74ACP is a well-known difficult sequence used
to test new synthesis protocols and used at Protein
Technologies, Inc. for quality control purposes.
ACP was synthesized with 2 × 30 sec deprotection
times and 2 × 1 min couplings for a total synthesis
time of 2.1 hr. HPLC analysis of the crude peptide
showed a significant prepeak due to incomplete
coupling of the valine (data not shown). However,
it was found that this prepeak could be eliminated
by coupling the valine in 1:1 dimethylformamide:
dimethylsulfoxide (DMF:DMSO) for 2 × 5
min (Figure 3).8

Figure 3 - HPLC of crude 65–74ACP. This 10-mer peptide was synthesized
in 2.1 hr with 2 × 30 sec deprotection times and 2 × 1 min coupling times.
G-LHRH
G-LHRH (H-GHWSYGLRPG-NH2) is a modified
version of the luteinizing hormone releasing hormone,
and is also used as a test peptide for quality
control of the Symphony and Prelude peptide
synthesizers. G-LHRH was synthesized in 2.3
hr using deprotection times of 2 × 30 sec and
coupling times of 2 × 1 min (Figure 4).
C-peptide
Chain A of the human proinsulin C-peptide9(H-EAEDLQVGQVELGGGPGAGSLQPLALE
GLG-OH) (Q is replaced with G)
was synthesized using reaction times of 2 ×
1.5 min and 2 × 2 min for deprotection and
coupling, respectively, in a total synthesis
time of 9 hr (Figure 5).
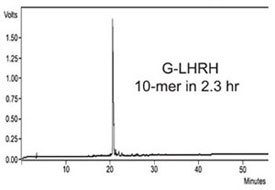
Figure 4 - HPLC of crude G-LHRH. This 10-mer peptide was synthesized
in 2.3 hr with 2 × 30 sec deprotection times and 2 × 1 min coupling times.
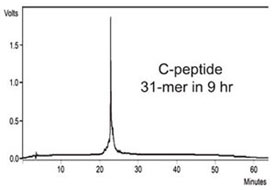
Figure 5 - HPLC of crude C-peptide. This 31-mer peptide was
synthesized in 9 hr with 2 × 1.5 min deprotection times and 2 × 2 min
coupling times.
Human amylin1–37
Human amylin1–37(H-KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTYNH2)
is a major component of the amyloid
deposits found in the pancreases of type-II
diabetes patients and contains a disulfide
bridge between Cys-2 and Cys-7.10 Linear
hAmylin1–37 was synthesized with deprotection
times of 2 × 1 min, and acylation
times of 2 × 2.5 min, resulting in a total
synthesis time of 10.8 hr (Figure 6a). Pseudoproline
dipeptides were incorporated
into the sequence using the Prelude’s Single-Shot delivery feature. Fmoc-Ala-Thr-ΨMe,Mepro-OH was coupled at position A8T,
Fmoc-Ser-Ser-ΨMe,Mepro-OH was coupled
at S19S, and Fmoc-Leu-Ser-ΨMe,Mepro-OH
was coupled at position L27S. The peptide
was then cyclized on the resin in 10 min by
treatment with thallium (III) trifluoroacetate
delivered by the Prelude’s Single-Shot delivery
feature, producing the cyclized peptide in
a total synthesis time of 11 hr (Figure 6b).

Figure 6 - HPLCs of crude a) linear and b) cyclized hAmylin1-37.
The 37-mer linear peptide was synthesized in 10.8 hr with 2 × 1 min
deprotection times and 2 × 2.5 min coupling times. It was then cyclized
in 10 min for a total synthesis time of 11 hr.
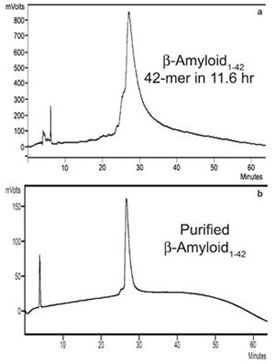
Figure 7 - HPLCs of a) crude and b) purified human b-amyloid1-42.
This 42-mer peptide was synthesized in 11.6 hr with 2 × 1 min deprotection
times and 1 × 5 min coupling times.
β-Amyloid1–42
Synthesis of the human β-amyloid1-42 peptide(H-DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA-OH) by conventional
SPPS has been reported to be difficult
due to on-resin aggregation and the high
hydrophobicity of the C-terminal segment.11
The authors synthesized β-amyloid1–
42 with
deprotection times of 2 × 1 min and acylation
times of 1 × 5 min for a total synthesis time of
11.6 hr (Table 1, Figure 7a). The peptide was
then purified by analytical HPLC (Figure 7b).
The HPLC product peaks were slightly broadened,
as seen before with this peptide.12
Conclusion
The authors demonstrated that HCTU is a
highly efficient coupling agent by using it to synthesize
seven peptides with deprotection times of
3 min or less and coupling times of 5 min or less.
Combining fast chemistry with peptide synthesizers
like the Prelude or Symphony, which have
been optimized for fast fluid deliveries, resulted
in cycle times as short as 14 min. Cost-savings
were realized using HCTU as a less expensive
activator, and minimizing reagent loss with the
Prelude’s Single-Shot delivery feature.
References
- Merrifield, R.B. Solid phase peptide synthesis 1. Synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–54.
- a) FastMoc™ chemistry: HBTU activation chemistry in peptide synthesis on model 430A 1990; Applied Biosystems. b) Fields, C.G.; Lloyd, D.H.; Macdonald, R.L.; Otteson, K.M.; Noble, R.L. HBTU activation for automated Fmoc solid-phase peptide synthesis. Peptide Res.1991, 4, 95–101. c) Schnolzer, M.; Alewood, P.; Jones, A.; Alewood, D.; Kent, S.B.H. In situ neutralization in Boc-chemistry solid phase peptide synthesis.
Int. J. Pep. Protein Res.1992, 40, 180–93. - Carpino, L.A. 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive. J. Am. Chem. Soc. 1993, 115, 4397–8.
- Alewood, P.; Alewood, D.; Miranda, L.; Love, S.; Meutermans, W.; Wilson, D. Rapid in situ neutralization protocols for Boc and Fmoc solid-phase chemistries. Meth. Enzymol. 1997, 289, 14–29.
- a) Marder, O.; Shvo, Y.; Albericio, F. HCTU and TCTU: new coupling reagents: development and industrial aspects. Chim. Oggi 2002, 20, 37–41. b) Sabatino, G.; Mulinacci, B.; Alcaro, M.C.; Chelli, M.; Rovero, P.; Papini, A.M. Assessment of new 6-Cl-HOBt based coupling reagents for peptide synthesis. Part 1: coupling efficiency study. Lett. Pept. Sci.2002, 9, 119–23. c) Sabatino, G.; Alcaro, M.C.; Pozo-Carrero, M.D.L.; Chelli, M.; Rovero, P.; Papini, A.M. Assessment of 6Cl-HOBt based coupling reagents in solid-phase cyclopeptide synthesis. In Peptides 2003; Chorev, M.; Sawyer, T.K., Eds.; American Chemical Society: Cardiff, CA, 2004; pp 49–50.
- Hood, C.A.; Fuentes, G.; Patel, H.; Page, K.; Menakuru, M.; Park, J.H. Fast conventional Fmoc solid-phase peptide synthesis with HCTU. J. Pep. Sci.2008, 14(1), 97–101.
- Muccioli, G.; Tschop, M.; Papotti, M.; Deghenghi, R.; Heiman, M.; Ghigo, E. Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. Eur. J. Pharmacol.2002, 440, 235–54.
- a) Fuentes, G.; Hood, C.; Page, K.; Patel, H.; Park, J.H.; Menakuru, M. Fast conventional synthesis of
65–74ACP on the Symphony® and Prelude™. European Peptide Symposium 2007; www.peptideinstruments.com/doc_files/doc_5.pdf (last accessed Mar 2008). - Heath, W.F.; Belagaje, R.M.; Brooke, G.S.; Chankce, R.E.; Hoffmann, J.A.; Long, H.B.; Reams, S.G.; Roundtree, C.; Shaw, W.N.; Slieker, L.J.; Sundell, K.L.; Dimarchi, R.D. (A-C-B) human proinsulin, a novel insulin agonist and intermediate in the synthesis of biosynthetic human insulin. J. Biol. Chem. 1992, 267, 419–25.
- Page, K.; Hood, C.; Patel, H.; Fuentes, G.; Menakuru, M.; Park, J.H. Fast Fmoc synthesis of hAmylin1–37 with pseudoproline assisted on-resin disulfide formation. J.Pep. Sci. 2007, 13, 833–8.
- a) Quibell, M.; Turnell, W.G.; Johnson, T. Preparation and purification of β-amyloid (1–43) via soluble, amide backbone protected intermediates. J. Org. Chem. 1994, 59, 1745–50. b) Tickler, A.; Clippingdale, A.B.; Wade, J.D. Amyloid-β as a “difficult sequence” in solid phase peptide synthesis. Protein and Peptide Letters2004, 11, 377–84.
- Burdik, D.; Soreghan, B.; Kwon, M.; Kosmoksi, J.; Knauer, M.; Henschen, A.; Yates, J.; Cotman, C.; Glabe, C. Assembly and aggregation properties of synthetic Alzheimer’s A4/beta amyloid peptide analogs. J. Biol. Chem. 1992, 267, 546–54.
The authors are with Protein Technologies, Inc., 4675 S. Coach Dr., Tucson, AZ 85714, U.S.A.; tel.: 520-629-9626; fax: 520-629-9806; e-mail: [email protected]. All tables and figures were adapted from Ref. 6.