Mercury is present in many areas of the environment, perhaps most notably in petrochemical products and fuels. Natural gas and its liquid condensates are primary feedstocks for a variety of industrial processes. It has long been known that petrochemical products contain significant levels of mercury and organomercury compounds. If they are not recognized and removed, these compounds can have disastrous effects.
Mercury in natural gas
Mercury occurs naturally in trace amounts in natural gas and natural gas condensate.1–4 (Although it is difficult to generalize, typical mercury concentrations are between 1 and 200 μg/m–3.) Mercury in natural gas condensate could be present in various forms (elemental, organometallic, and inorganic salt), depending on the origin of the condensates.5 Knowledge of the total mercury content and the different species present in natural gas condensate is extremely important. First, mercury in most forms is highly toxic; particularly when present as the organomercury species, it is a cause of great environmental concern. Second, the damage caused to industrial facilities, particularly petrochemical plants, by the presence of mercury species can be financially crippling. At least five cases exist in which mercury contamination from natural gas resulted in significant facility damage, with a resultant shutdown.
The implication of the effect of mercury in natural gas was not reported until 1973, when a catastrophic failure of an aluminum heat exchanger occurred at the Skikda Liquefied Natural Gas Plant in Algeria, Africa.4–6 Subsequent investigations determined that mercury corrosion caused the failure; however, the source of mercury was in debate.4,7 After similar plant failures in other gas fields, the full mercury problem was realized together with the multimillion-dollar cost implications.
Frech8 defined the problem thus: “At present it is not well known in which chemical forms mercury is present in natural gases and gas condensates and, in addition, methods for the determination of total mercury concentrations must be regarded to be of unproven reliability due to lack of adequate standard reference materials and poor accuracy.” While a number of workers have addressed this problem in terms of the speciation,9,10 quantitative approaches for total mercury determinations in such samples are vital.
For several years, in association with both academic and industrial partners, the author has been developing analytical procedures and instrumentation using atomic fluorescence spectrometry (AFS) to determine mercury in its various forms at very low levels in the environment, especially in difficult matrices such as petrochemicals.
Atomic fluorescence is a spectroscopic process that is based on the absorption of radiation of a certain wavelength by an atomic vapor and subsequent radiational deactivation of the excited atoms toward the detection device. Both the adsorption and the subsequent atomic emission processes occur at wavelengths that are characteristic of the atomic species present. Atomic fluorescence spectrometry is a very sensitive and selective method for the determination of a number of environmentally and biomedically important elements, such as mercury, arsenic, selenium, bismuth, antimony, tellurium, lead, and cadmium.
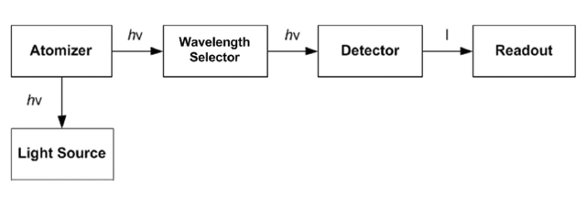
Figure 1 - Schematic diagram of atomic fluorescence spectrometry instrument.
The basic layout of an AFS instrument is shown in Figure 1. It is similar to atomic absorption spectrometry (AAS), except that the light source and detectors are placed at right angles.
A number of excitation sources have been used in atomic fluorescence spectrometry, primarily spectral line sources and continuous sources. For the determination of mercury, a low-pressure mercury discharge lamp provides a simple, intense source suitable for these measurements.
For any analytical procedure, there are several aspects of the cycle that need to be addressed and controlled. Certainly, the most important aspect is the sampling itself, ensuring that a fully representative sample has been taken that has not been compromised in any way or form. This is particularly important in the petrochemical industry, where the matrix ranges from volatile gaseous materials to heavy condensates. The sampling and the attention to detail required to meet safety requirements are of considerable importance in determining a suitable analytical procedure.
For the determination of mercury, there are two fundamental parts of the measurement cycle. First is the chemical process of generating the analyte in vapor form, and second is quantification of the analyte using a specific atomic fluorescence spectrometry instrument.
Continuous-flow vapor generators have been commercially available for many years. The sample and reagent streams and all gaseous products are continuously and rapidly pumped into a glass gas–liquid separator, from which the gaseous products are carried, by argon gas, to the atomic fluorescence spectrometry detector.
The design of the atomic fluorescence spectrometry detector for mercury determination is relatively simple because of the absence of a thermally energized atomizer.11 Generally, a UV mercury vapor lamp is used as excitation source, and the fluorescence light is detected by a photomultiplier tube, which is positioned perpendicular to the excitation source. Figure 2 illustrates the optical configuration of an AFS system.12

Figure 2 - Schematic diagram of continuous-flow vapor generator, illustrating the optical configuration of an AFS system for mercury determination.
Since the system is used to determine total inorganic mercury (Hg2+), the first requirement for performing the analysis is to liberate all mercury compounds from the sample matrices and convert all organic forms of mercury to Hg2+ by various digestion/oxidation procedures.
Once all forms of mercury have been converted to Hg2+, the latter is reduced to elemental mercury (Hg0) using either acidified or alkaline stannous chloride:
Hg2+ + Sn2+ = Hg0 + Sn4+
The mercury vapor produced is carried by argon gas to the atomic fluorescence spectrometry instrument for detection.
Mercury in water
In order to determine all forms of mercury in water samples by vapor-generation atomic fluorescence spectrometry, it is necessary to treat the samples to oxidize all forms of mercury that may be present in the sample to inorganic Hg2+. This step usually takes 30 min to perform and involves the manual addition of HCl and Br–/BrO3– reagents. An alternative to this is to perform the treatment on-line, thus reducing the potential sources of error, as well as reducing tedious sample preparation steps. Assuming the sample has been acidified at the point of sampling, then nothing else needs to be done to the sample.
The system involves the coupling of the mercury analyzer with a UV cracker and associated cooling modules. The sample is mixed with an oxidant stream of acidified bromide/bromate, which then passes through the UV digestion system. Here, the UV increases the rate of the bromination reaction, allowing the oxidation of organomercury to be performed rapidly. The oxidized mercury then flows through a cooling module before it goes into a T-piece, where it mixes with a reductant, usually acidified SnCl2, to form mercury vapor, which is carried with argon from a gas–liquid separator, through a drying membrane, into the detector. A fluorescence signal proportional to the mercury concentration is obtained.
Use of a UV oxidation system for the on-line treatment of mercury by vapor generation eliminates the need for time-consuming sample pretreatment, in addition to eliminating contamination from flasks, pipets, reagents, etc. Samples can be analyzed without any sample pretreatment. This has several advantages because sample pretreatment stages are frequently the most time-consuming part of analyses and are also major sources of errors caused by contamination. Since drinking water usually has mercury concentrations below 10 ppt, it is essential that possible sources of contamination be avoided.
In some instances, such as the determination of mercury in pristine waters, lower detection limits than even atomic fluorescence spectrometry alone can offer are necessary. To lower the detection levels, the mercury evolved from the samples is first purged from the gas–liquid separator and passed over an Au/Pt gauze, which subsequently collects the mercury by amalgamation. After the collection stage, the mercury is released by thermal desorption and the resulting mercury is transported to the detection system. The exact sequence of events can be fully automated and is available commercially.13
Mercury in coal
New regulations in many countries, including the U.S., require all coal-fired power stations over 420 MW to monitor their emissions for mercury on a continuous basis. The main source of mercury in such emissions is the coal used to run the plant; it is therefore advantageous to be able to monitor mercury levels in the coal to try to minimize stack gas emissions.
A method for the determination of mercury contained in coal samples using vapor-generation AFS following an aqua regia digestion of the sample has been developed. Coal samples were accurately weighed and transferred into acid-washed digestion vessels containing aqua regia and heated at 140 °C for a period of 2 hr. Once cooled, the samples were filtered and diluted with deionized water. The samples were then analyzed using a continuous-flow vapor-generation AFS system.
The results in Table 1 show both good agreement with the certified value and good precision. All results are based on duplicate preparation and duplicate analysis (i.e., n = 4). The fact that the analytical peak profile of the sample is similar to that of the standards is indicative that no matrix interferences were present.
Several of the cited references refer to instrumentation that has been built in research departments, while others have coupled commercially available AFS instruments to commercial chromatographic systems. To the author’s knowledge, AFS instruments for the analysis of mercury and some other elements are currently being manufactured by P S Analytical Ltd. (Orpington, Kent, U.K.), Brooks Rand Inc. (Seattle, WA), and Tekran Inc. (Toronto, Ontario, Canada), and by these companies, all located in Beijing, China: Beijing Titan Instrument Co., Beijing Rayleigh Analytical Instrument Corp., East & West Analytical Instruments, and Beijing Kechuang Haiguang Instrument Co. Ltd.
Determination of mercury levels in liquid and gas streams
In the petrochemical industry, the hydrocarbons present range from gaseous streams to fully liquid streams, and it has been previously ascertained that mercury can be present in all of these fractions. The techniques for determining the levels of mercury present and the strategies for its removal will be influenced by the form of these fractions. In addition, the safety requirements of any production site will also determine the construction of any process instrumentation. The determination of mercury in these fractions requires unique approaches, particularly regarding the need to provide a fully representative sample for the measurement stage. Generally speaking, the procedure developed for gaseous samples provides the cornerstone for designing the instrumental system, since heavier materials can be vaporized and subsequently treated as gaseous.

Figure 3 - Schematic diagram of sampling system for the determination of mercury in natural gas: V = isolation valve on pipeline, V1 = isolation valve for 10.537, P1 = press gauge for pipeline, PVR1 = heated pressure regulator, P2 = pressure gauge prior to sampling tubes, and FM = wet gas flow meter.
The author has developed an off-line sampling device to ensure that a representative sample is collected (Figure 3). The unit has been designed to operate in Hazardous Zone 1 areas using ExDIICT3-rated components. This enables the samples to be collected at the pipeline and therefore minimizes any contamination or losses often found when high-pressure PTFE-lined cylinders have been used. The experience obtained using this off-line procedure has ensured that on-line continuous systems can be designed to operate reliably and accurately in process conditions.

Figure 4 - Schematic diagram illustrating the automated thermal desorption cycle. Samples collected remotely on trap B are transferred to trap A for measurement.
To overcome condensation of hydrocarbons on the sampling tube, the adsorbent is maintained at 140 °C, which is well above the dewpoint of the hydrocarbon gas. The trapping efficiency of the Amasil™ tube (P S Analytical) was studied by Dumarey et al.14 for a wide range of mercury compounds, and in all cases excellent trapping efficiency was obtained.
The sample collection tube can be used at elevated temperatures of up to 200 °C without breakthrough. This enables reliable collection of mercury from wet natural gas streams. Corns et al.15 have shown that the Amasil trap collects all known forms of mercury, even at elevated temperatures up to 210 °C.
The instrumentation operates on the principle of dual amalgamation coupled to AFS. The automated thermal desorption arrangement is shown in Figure 4. Prior to sampling, the sampling tubes are cleaned to give a reproducible blank.
The clean sample tubes are then taken to the sampling point and inserted into the sample tube furnace. The natural gas is then passed over the tube until a sufficient volume of gas has passed over for reliable quantification. The detection limit for the instrumentation is 0.1 pg, which equates to 1 ng/m3 for a 10-L sample volume, and the upper working range is 300 μg/m3. The wide linear dynamic range of the Sir Galahad allows relatively small sample volumes to be collected, thus minimizing the time required for sampling. The system has been used on various gas refineries around the world and has become invaluable when monitoring the efficiency of mercury removal units.16

Figure 5 - a) Configuration of GC-AFS instrument; b) schematic block diagram of GC-AFS.
The author has further developed the technology to expand the product range analyzed by the atomic fluorescence technique. From liquefied natural gas to liquid hydrocarbons17 to heavy condensates, the analytic approach outlined has proved successful, and fully automated continuous on-line systems have been designed, constructed, and installed at customer sites. The procedure whereby samples are collected into clean gold traps and excess hydrocarbons removed prior to vaporization of the mercury into the detector module, and the ready availability of a traceable standard mercury source, ensures reliable, long-term operation on-site. Site safety criteria also increase the complexity of the installed system, and meeting these criteria adds greatly to the investment in time and money.
Mercury speciation in liquid hydrocarbons
Although the focus here has been on the determination of mercury in various petrochemical fractions, the composition of the mercury content (i.e., speciation) is also of considerable importance. For such measurements, the value of the atomic fluorescence measurements cannot be underestimated. Stockwell et al.18,19 have described a specific gas chromatography system coupled to atomic fluorescence to determine the individual species in the matrix. The capillary gas chromatograph-atomic fluorescence spectrometer (GC-AFS) instrument (Figure 5a) has been used for a number of years, mainly for environmental applications, in which organomercury compounds are extracted from the sample prior to injection.20 Figure 5b shows a schematic block diagram of the configuration currently in use.
For the determination of organomercury in liquid hydrocarbons,21,22 no sample preparation was required. On-column injection was used to introduce the sample. The individual organomercury compounds exiting the column are pyrolyzed at 800 °C to convert them to mercury vapor, which is subsequently delivered to the AFS detector with the aid of an argon make-up gas. Adding a pyrolyzer to the column outlet and diverting the flow to the AFS detector can be readily accomplished.
Conclusion
The application of AFS to the measurement of mercury has been described. The sensitivity of the measurement technique makes it an ideal facilitator for these measurements. However, the analytical techniques are not the sole determinant of a reliable system, and the sampling and sample pretreatment illustrated in this article ensure that reliable data are provided, allowing process operators to control the processes and avoid costly plant shutdowns.
References
- Bingham, M.D. SPE Prod. Eng. May 1990, 120–4.
- Haselden, G.G. Mech. Eng. 1981, 103, 46.
- Bodle, W.W.; Attari, A. et al. Paper presented at Institute of Gas Technology Sixth International Conference on Liquefied Natural Gas, Kyoto, Japan, Apr 7–14, 1980.
- Leeper, J.E. Energy Process Can. 1981, 73, 46–51.
- Arsenic and Mercury Removal in Natural Gas, Refining and Petrochemical Industries. IFP Tech. Bull. 1983, 1–10.
- Leeper, J.E. Hydrocarbon Process 1980, 59, 237.
- Frech, W.; Baxter, D.C. et al. J. Anal. At. Spectrom. 1995, 10, 769.
- Frech, W.; Baxter, D.C. et al. Anal. Commun. 1996, 33, 7.
- Snell, J.; Frech, W. et al. Analyst1996, 121, 1055.
- Snell, J.; Qian, J. et al. Analyst 1998, 123, 905.
- Cai, Y. Atomic Fluorescence in Environmental Analysis. In: Encyclopaedia of Analytical Chemistry, Meyers, R.A., Ed.; John Wiley & Sons Ltd.: New York, 2001, pp 1–22.
- Olafsson, J. A report on the ICES intercalibration of mercury in seawater for the Joint Monitoring Group of the Oslo and Paris Commissions. Marine Chem. 1982, 11, 129–42.
- SA10.035. Millennium Merlin User Manual, v4, Sep 2010, P.S. Analytical, Orpington, Kent, U.K.
- Dumarey, R.; Dams, R. et al. Anal. Chem. 1985, 57, 2638.
- Corns, W.T.; Stockwell, P.B. et al. J. Anal. At. Spectrom. 1993, 8, 71–7.
- PSA10.525. Sir Galahad II User Manual, v1, Nov 2010, P.S. Analytical, Orpington, Kent, U.K.
- Stockwell, P.B. Atomic Fluorescence Spectrometry: An Ideal Facilitator for Determining Mercury and Arsenic in the Petrochemical Industry. In: Spectroscopic Analysis of Petroleum Products and Lubricants, Kishore Nadkarni, R.A., Ed.; ASTM International: West Conshohocken, PA, 2010; Chap. 10, pp 246–86.
- Stockwell, P.B.; Corns, W.T. Hydrocarbon Asia Oct 1993, 36–41.
- Stockwell, P.B.; Corns, W.T. Oil Gas Sci. Technol. Now 1994, Autumn, 58–61.
- Alli, A.; Jaffe, R. et al. J. High Res. Chromatogr. 1994, 17, 745.
- Shafawi, A.; Foulkes, M.E. et al. Analyst 1999, 124, 185–9.
- Shafawi, A.; Foulkes, M.E. et al. J. Anal. At. Spectrom. 1999, 14, 1245–85.
Peter B. Stockwell, Ph.D., is with P S Analytical, Arthur House, Crayfields Industrial Park, Main Rd., Orpington, Kent BR5 3HP, U.K.; tel.: +44 1689 891211; fax: +44 1689 896009; e-mail: pbs@ psanalytical.com.