Nanoparticle analysis is becoming a central part of pharmaceutical development. For example, molecular aggregration, which leads to the formation of nanoparticles and microparticles, can render carefully constructed drug formulations worthless and interfere with a drug’s safety, bioavailability and effectiveness. In addition to the pharmaceutical industry, nanoparticle analysis finds application in drug delivery, disease detection, food production, cosmetics, coatings and environmental monitoring.
Quantitative instruments are required that are able to detect nanoparticles with diameters below roughly one micron, especially at the level of polydispersity and concentrations of interest in pharmaceutical and related applications. Dynamic light scattering (DLS) is used for this purpose, mainly due to its ease of use and ability to detect a wide range of particle sizes. However, DLS cannot reliably measure polydisperse solutions, instead sometimes reporting a single peak in a particle distribution when in fact there are multiple, separate groups of particle diameters. In addition, the technique cannot provide concentration information, which is often a critical parameter in monitoring or debugging a process.
Other emerging light-scattering technologies can track and size individual particles, circumventing the challenges DLS encounters with polydisperse samples. Yet these technologies still suffer from the limitations associated with the index-contrast requirement inherent to the fundamental operating principle: particles with an optical index close to that of the suspension medium scatter much less light than those with a significantly different index, leading to a bias toward “brighter” particles and an inability to detect some types of particles. As a result, many biological particles below 100–200 nm in diameter cannot be measured. Further, these techniques work only in a narrow range of concentrations, and the software must be able to distinguish and properly track particles. Single-particle optical tracking thus provides an interesting but somewhat unproven complement to the much more accepted DLS technology.
The nCS1 (Spectradyne LLC, Torrance, Calif.) measures the size distribution of nanoparticles with diameters ranging from 30 nm up to a few microns, over a range of concentrations from 107 to 1012 particles/ mL. Size distributions of a wide variety of nanoparticles in a range of suspension media are accurately reported, as are data in absolute nanoparticle concentration units.
The technology* is based on the extreme miniaturization of the resistive pulse sensing technique employed by the Coulter Counter: an analyte solution carrying the nanoparticles or microparticles of interest is passed through a narrow constriction a few hundred nanometers to a few microns in diameter (see Figure 1). The suspending solution is weakly electrically conducting, meaning it has a few tens to a few hundred mM salt dissolved in it, giving it an electrical conductivity on the order of 1 S/m. When a nanoparticle passes through it, the electrical conductivity of the solution in the nanoconstriction decreases, as the nanoparticle occludes the electrical current. This change in conductance is closely proportional to the nanoparticle volume, so monitoring the conductance as individual particles pass through the constriction yields the volume of each particle. The raw data comprise conductance pulses in time, whose amplitude is directly proportional to the individual particle volume, and whose duration gives the particle speed. As the particles are entrained in the suspending fluid, the pulse duration also gives the fluid speed; calibrations based on the details of the nanoconstriction geometry then give the volumetric flow rate, enabling calculation of the concentration of particles.
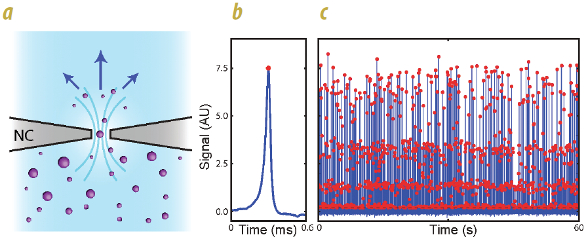 Figure 1 – a) Schematic of the Coulter principle, in which nanoparticles pass through a narrow constriction (nanoconstriction, or NC), occluding the electrical conductance of the suspending solution and increasing the electrical resistance through the nanoconstriction. b) Data comprise resistance pulses in time, where the size of the resistance change indicates particle volume and duration indicates particle and suspending fluid velocity. c) Accumulating sufficient pulses in time; fitting the height (red points) and width of the pulses yields the distribution of particle volumes and the concentration. The vertical distribution of red points represents the dispersion of each particle diameter in the mixture, and concentration is obtained from the calculated flow rate of the suspension from the pulse widths.
Figure 1 – a) Schematic of the Coulter principle, in which nanoparticles pass through a narrow constriction (nanoconstriction, or NC), occluding the electrical conductance of the suspending solution and increasing the electrical resistance through the nanoconstriction. b) Data comprise resistance pulses in time, where the size of the resistance change indicates particle volume and duration indicates particle and suspending fluid velocity. c) Accumulating sufficient pulses in time; fitting the height (red points) and width of the pulses yields the distribution of particle volumes and the concentration. The vertical distribution of red points represents the dispersion of each particle diameter in the mixture, and concentration is obtained from the calculated flow rate of the suspension from the pulse widths.The resistive pulse sensing technique works regardless of whether the nanoparticles are made of insulating or conducting material, so a given diameter polystyrene or colloidal particle will provide the same signal as the same diameter gold or other metal particle. This is because the voltage drop required to pass an electrical current through the nonlinear electrochemical barrier formed by the solution around a conducting particle is larger than the small sense voltage applied across the nanoconstriction, and much larger than the voltage drop across the particle itself. Spectradyne has verified that nCS1-based measurements of diverse particle types (e.g., metal versus insulating) are in agreement with other techniques such as transmission electron microscopy (TEM).
In the nCS1 measurement, the nanoconstriction part of a microfluidic circuit forms the heart of a disposable, single-use cartridge (see Figure 2). The analyte of interest is pipetted into a reservoir in the cartridge, which is then placed in the instrument for measurement. Only 3 μL of analyte is required, and the analyte does not come into contact with other instrument components prior to analysis, removing the possibility of cross-contamination with other samples. Automated software then controls operation of the electronics and microfluidics, and a statistically significant data set can be generated about one minute after inserting the cartridge into the instrument.
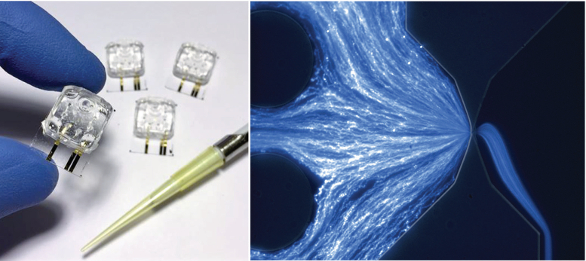 Figure 2 – Disposable microfluidic cartridge for nanoparticle analysis (left); image of fluorescent nanoparticles flowing through a cartridge nanoconstriction (right).
Figure 2 – Disposable microfluidic cartridge for nanoparticle analysis (left); image of fluorescent nanoparticles flowing through a cartridge nanoconstriction (right).Following data acquisition, the instrument software rapidly generates size histograms in which particle distribution is reported as an absolute concentration spectral density (CSD), with units of number of particles per milliliter analyte solution per nanometer particle diameter (see Figure 3). A software integration tool can report the absolute concentration of particles in a given range of particle diameters and generate size histograms with user-defined diameter bin widths, with units of particles per milliliter. Examples of the integrated concentrations are also given in Figure 3. This concept will be familiar to users of electrical spectrum analyzers, which report electrical spectral power densities in units of W/Hz, where the power contained in a band of frequencies is obtained by integrating the power spectral density over the desired range of frequencies, yielding the absolute power in units of W.
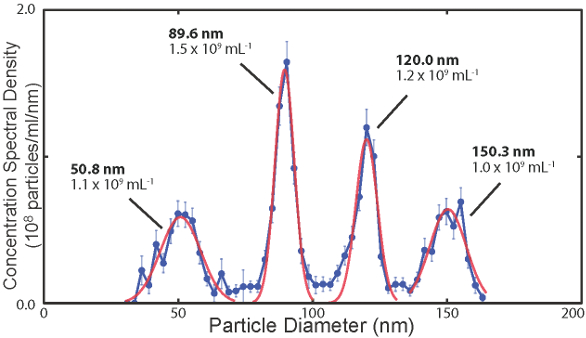 Figure 3 – Concentration spectral density (CSD) of a polydisperse particle distribution, where the vertical axis gives the number of particles per mL per nm of diameter. Data were obtained by analyzing the time-based data shown in Figure 1. The red curves are fits to the distribution, and the concentrations of particles listed under each mean diameter are obtained by integrating the fits over the relevant range of diameters associated with each particle population, yielding number of particles per mL.
Figure 3 – Concentration spectral density (CSD) of a polydisperse particle distribution, where the vertical axis gives the number of particles per mL per nm of diameter. Data were obtained by analyzing the time-based data shown in Figure 1. The red curves are fits to the distribution, and the concentrations of particles listed under each mean diameter are obtained by integrating the fits over the relevant range of diameters associated with each particle population, yielding number of particles per mL.The cartridge’s microfluidic circuit is cast from a reusable mold. Using NIST-traceable particle standards to measure the consistency of cartridge performance, diameter measurements agree to within a few percent for cartridges cast from the same mold. This repeatability provides an efficient means with which to establish secondary calibration of the mold and all cartridges made from it. Complex microfluidic circuits can be cast using this technology. Details of the cartridge design can be found in Ref. 1.
Conclusion
The nCS1 brings the reliability of resistive pulse sensing to the analysis of nanoparticles, with a cartridge-based analysis format that eliminates concerns about cross-contamination.
Reference
- Fraikin, J.L.; Teesalu, T. et al. A high-throughput label-free nanoparticle analyser. Nature Nanotechnol. 2011, 6, 308–13; U.S. Patent 8901914.
Andrew N. Cleland is scientific and technical advisor, Jean-Luc Fraikin is chief scientist, Peter Meinhold is chief engineer and Franklin Monzon is chief executive officer, Spectradyne LLC, 23875 Madison St., Suite A, Torrance, Calif. 90505, U.S.A.; tel.: 626-390-8530; e-mail: [email protected]; www.spectradynellc.com. Andrew N. Cleland is John A. MacLean Sr. Professor for Molecular Engineering Innovation and Enterprise at the University of Chicago, Chicago, Ill., U.S.A. Peter Meinhold is an associate research physicist at the University of California, Santa Barbara, Calif., U.S.A.
*Spectradyne’s technology was developed at, and has been licensed from, the University of California at Santa Barbara. The underlying technology, however, has been in use for over 60 years. It originated in Wallace Coulter’s 1949 patent (U.S. Patent no. 2656508) and provides an established standard for the analysis of micron-scale particles, for example, in whole-blood cell counting. Spectradyne’s patented technology allows scaling to a microfluidic technology that can measure particle diameters approaching 10 nm, and detecting such small particles at very high count rates—on the order of 104 particles/sec—so that statistically significant data sets can be accumulated in seconds.