
Figure 1 - Multiple photovoltaic solar panels for energy generation.
Research into alternative energy sources is accelerating, with one of the most dynamic areas of interest being the development of devices to harness solar energy. Some industry experts, including the authors of “A Solar Grand Plan,” believe that 69% of total electricity in the U.S. could be sourced from solar power by the year 2050, given the necessary level of investment, thereby ending U.S. dependence on fossil fuels and slashing greenhouse gas emissions.1 Harnessing the sun’s power requires highly efficient energy converters (photovoltaic cells) (Figure 1), the majority of which are based on silicon. Impurity control of solar-grade silicon, particularly for boron and phosphorus, is critical to the efficiency of the finished device. Historically, the analysis of phosphorus in silicon has been performed by inductively coupled plasmaoptical emission spectroscopy (ICP-OES), but with a demand for lower detection limits (DLs), a more sensitive method is needed.
Analytical challenges
The latest industry requirement for impurity levels in silicon is lower than 10 ppb in the solid. Boron and phosphorus are of particular importance, but are difficult elements to analyze using ICP-MS. In a silicon matrix, the polyatomic interference 30Si1H interferes with 31P. An alternate strategy is to determine phosphorus indirectly by measuring 31P16O at mass 47, but here 28Si19F also interferes (see “Recovery Test” section for more detail). Boron is a volatile element that is easily lost during the sample preparation stage. It is critical, therefore, that a new method removes silicon for the determination of phosphorus, while avoiding the loss of boron.
Silicon sample
Pieces of silicon taken from a block of silicon were analyzed for the following analytes: Li, B, Na, Mg, Al, K, Ca, P, Ti, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, As, Sr, Zr, Nb, Mo, Cd, Sn, Sb, Ba, Ta, W, Pb, Th, and U. The method can also be applied to polysilicon granules or chips.
Sample preparation
Two sample preparation procedures were followed: one for the analysis of all elements except boron, and a slightly modified procedure for boron. First, the sample surface was cleaned with hydrofluoric acid (HF). The sample pieces were then dissolved in HF/HNO3. A small aliquot of H2SO4 was added, except to the samples being prepared for boron analysis (the higher temperature generated as the water is evaporated due to the presence of H2SO4 would result in the loss of volatile boron). Sample solutions were heated to near dryness, and then dissolved in 0.5 w/w HNO3.
Calibration standard solution
The calibration blank solution contained 0.34% (w/w) HNO3 and 0.33% (w/w) H2SO4. Calibration standards were prepared by spiking the blank solution with a multielement standard solution (SPEX CertiPrep Inc., Metuchen, NJ) at the following concentrations: 0, 0.1, 0.2, 0.5, and 1 ppb.
Instrumentation
An Agilent 7500cs ICP-MS with Octopole Reaction System (ORS) (Agilent Technologies, Palo Alto, CA) was used throughout the study. The ORS is a collision/reaction cell (CRC) that is used to attenuate polyatomic ions that interfere with elements of interest. When helium (inert collision gas) is added to the ORS, polyatomic interferences are removed using a process known as kinetic energy discrimination (KED). The larger interfering species experience more collisions with helium atoms and lose energy as they pass through the cell. An energy differential is applied to prevent the lower-energy polyatomic ions from entering the mass filter.
When a reaction gas is added to the ORS, e.g., hydrogen, interferences are neutralized or converted to another species by reaction. For interference-free analytes, the cell can be operated in either helium collision or no-gas mode, i.e., with no gas added to the ORS cell.
Recovery test
Table 1 - Concentrations in solution (ppb) and recovery data for 5-ppb spikes added to silicon samples prepared with and without the addition of H2SO4

When silicon is dissolved in HF/HNO3, a significant amount of silicon remains in solution, even after heating. Phosphorus determination at mass 31 is normally performed by operating the ORS in helium collision mode. However, any silicon present in solution creates 30SiH in the plasma, which interferes with 31P; 14N16OH also overlaps at mass 31. Another approach for the determination of phosphorus is to measure the PO (phosphorus monoxide) polyatomic ion at mass 47, which can be produced using cool plasma conditions (reduced plasma power). In this case, the background equivalent concentration (BEC) for phosphorus is very low at 20 ppt. Although titanium has an isotope at mass 47, it is not ionized under cool plasma conditions, so there is no interference with PO. Conversely, titanium can be measured using helium collision mode under normal plasma conditions because PO does not form in a hot plasma. Using a combination of measurement modes, both PO and titanuim can be determined effectively. It is worth noting that because any silicon remaining in solution with HF will give rise to 28Si19F, which interferes with 31P16O, complete elimination of silicon from the sample solution is absolutely necessary in order to determine phosphorus, regardless of the measurement mode used. This is achieved by the addition of H2SO4. However, when heating the sample solution with H2SO4, the high temperature results in the loss of boron as BF3. Therefore, a slightly modified, lower-temperature sample preparation without the addition of H2SO4 (since complete removal of the silicon matrix is not required), is used for boron. A recovery test was performed to validate this sample preparation strategy; results are given in Table 1.
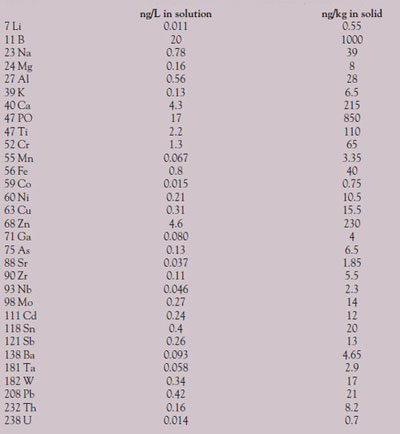
Table 2 - Detection limits (3 sigma) in solution and in the solid (all units ppt)
From the recovery test results, it can be concluded that the addition of sulfuric acid removes the silicon matrix completely (as well as boron), due to the higher-temperature evaporation process, which makes it possible to determine phosphorus at the low-ppb level in the solid. The results also show that a simple evaporation without sulfuric acid is effective for the determination of boron. Although it has been reported that the addition of mannitol stabilizes boron in solution, acceptable recoveries can be obtained without the addition of mannitol, provided high solution temperatures are avoided. With no H2SO4 added, it is important to closely monitor the evaporation of the sample. If the sample is allowed to evaporate to dryness, Nb, Ta, W, and Au will be lost. The concentration of silicon remaining in the non-H2SO4 preparation solution was 10–20 ppm.
Detection limits
Three-sigma DLs were calculated from the calibration curves, as shown in Table 2. DLs in the solid were obtained by multiplying the solution DL by 50 (0.3 g made up to 15 mL).
Conclusion
Ultratrace elemental impurities in solar-grade silicon can be analyzed successfully with the Agilent 7500cs ICP-MS. Boron and phosphorus, which are key elements in this application, can be determined at the low-ppb level in the solid, and other elements studied can be measured at the ppt level.
Reference
- Zweibel, K.; Mason, J.; Fthenakis, V. A solar grand plan. Sci. Am. Jan 2008.
Dr. Takahashi is with Agilent Technologies, 9-1 Takakura-cho, Hachioji, Tokyo 192-0033, Japan; tel.: +81 426 60 9935; fax +81 426 60 8656; e-mail: [email protected].