Formaldehyde or formaldehyde-releasing preservatives are frequently used in cosmetic preparations such as shampoos and skin-care products for the prevention of microbial and fungal growth. Many countries, including the European Union, have applied restrictions on the concentration of formaldehyde measured in the free form in such products.1 Accurate determination of free formaldehyde concentration is essential for correct labeling and classification of many products in accordance with these regulations.
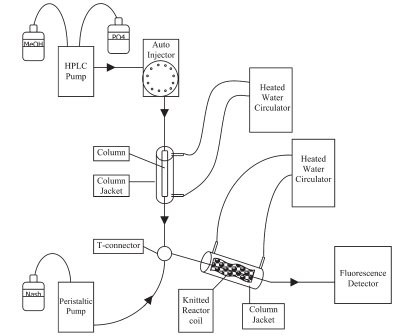
Figure 1 - Schematic of the configuration of the HPLC postcolumn detection system used in the application.
In the HPLC method used here, free formaldehyde elutes as an early peak (1.83 min) Nash reagent2 through a postcolumn derivatization reaction (formaldehyde, as well as ammonium acetate, acetylacetone, glacial acetic acid, butyraldehyde, and proprionaldehyde, were obtained from Sigma-Aldrich, St. Louis, MO). A schematic of the HPLC postcolumn derivatization setup is shown in Figure 1. For clarification, it is generally accepted that formaldehyde in its hydrated form of methylene glycol and polymethylene glycol is derivatized to completion with reagents such as 2,4-dinitrophenylhydrazine (DNPH) and Nash reagent. It is assumed, therefore, that the term “free formaldehyde” refers to monomeric formaldehyde, methylene glycol, and polymethylene glycol.2 This method was found to be specific for formaldehyde and gave no response with acetaldehyde, propionaldehyde, buteraldehyde, or benzaldehyde.

Figure 2 - Calibration curve of formaldehyde using postcolumn chemical derivatization with Nash reagent (n = 3).

Figure 3 - Chromatograms of a) formaldehyde, 1000-ppm dilution; b) Glydant, 10,000-ppm solution, formaldehyde concentration: 734 ppm; c) Glydant XL-1000,10,000-ppm solution, formaldehyde concentration: 1020 ppm; d) Germall® 115 (Sutton Laboratories, Chatham, NJ), 10,000-ppm solution, formaldehyde concentration: 420 ppm; e) Stepanol AM, 10,000-ppm solution, formaldehyde concentration: 2 ppm; f) Stepanol WAT, 20,000-ppm solution, formaldehyde concentration: 2.5 ppm;and g) Dowicil™ 200 Preservative (Dow Chemical, Midland, MI), 10,000-ppm solution, formaldehyde concentration:101 ppm. Retention times for each sample are shown on the graph. Note the difference of scale on they-axis for e, f, and g.
A calibration curve was determined for formaldehyde (Figure 2) under the stated conditions. The calibration curve follows a polynomial function (R2 = 0.9985). Four commercial cosmetic preservatives and five cosmetic surfactants were assayed for formaldehyde. The concentration of formaldehyde was calculated using the polynomial formula derived from the calibration curve (Figure 2). No detectable level of free formaldehyde was found in the following surfactants: Steol® CS-370, Steol CS-270, and Stepanol® WAT-K (all Stepan Co., Northfield, IL). The formaldehyde concentrations of the six remaining samples ranged from 2 ppm (Stepanol WAT) to 1084 ppm (Glydant® XL-1000, Lonza Inc., Allendale, NJ) (Figure 3). Dowicil 200 Preservative displayed an initial formaldehyde peak at 1.83 min at a concentration of 100 ppm, but also displayed a second peak at 3.02 min. This was most likely additional formaldehyde being released more slowly from the Dowicil molecule (Figure 3g). The lower limit of detection of free formaldehyde using the stated method is 0.7 ppm (calculated as three times the background noise). Twelve replicate samples of 500 ppm formaldehyde were run; the calculated percent standard deviation of response and retention time were 1.70% and 0.34%, respectively.

Figure 4 - EPOCOD EP-B-4-5-50.
This improved methodology for the analysis of free formaldehyde in cosmetic preparations employs several cost-effective changes to existing protocols. The use of a peristaltic pump as part of a standard EPOCOD™ (economical postcolumn derivatization kit, Aura Industries, New York, NY) to deliver the postcolumn chemical derivatization agent provides a consistent flow rate that would otherwise have required an additional HPLC pump (Figure 4). Costly acetonitrile solvent, which is more commonly used for this type of procedure,2 has been replaced with methanol. Finally, Poland Spring® brand distilled water (Wilkes Barre, PA) is used for reagent, sample, and eluent preparations rather than much more expensive HPLC-grade water.
Materials and methods
The HPLC parameters are shown in Table 1. The postcolumn conditions were as follows: A Masterflex peristaltic pump (Thermo Fisher Scientific, Waltham, MA) was employed in conjunction with an EPOCOD postcolumn derivatization kit and 5-50 KRC knitted reactor coils (5m × 0.5 mm i.d i.d.) (Aura Industries). The flow rate was 0.8 mL/min and the heated reactor temperature was 70 °C.
Derivatization with Nash reagent: 77 g of ammonium acetate was dissolved in roughly 250 mL of Poland Spring brand distilled water; 1.25 mL of glacial acetic acid and 1 mL of acetylacetone were added. After vigorous mixing, the solution was transferred to a 0.5-L volumetric flask, followed by 200 mL of water. The pH of the solution was adjusted to 5 with glacial acetic acid, and the mixture was diluted to the 0.5-L mark with water. The solution was then filtered through an IFDA filter degasser (Aura Industries) with PTFE filter membrane (catalog no. TFM 1.5- 10), ~1.5-µm pore size (Aura Industries).
For detection, the model 980 programmable fluorescence detector (Applied Biosystems, Foster City, CA) was used, with 550-nm emission filter and 410-nm excitation wavelength.
Sample preparation
Formaldehyde (37% in water/methanol) was diluted to concentrations ranging from 2000 ppm to 1 ppm with Poland Spring brand distilled water:methanol (85:15). Butyraldehyde and proprionaldehyde were diluted to 2500 ppm with 85:15 water:MeOH solution, and benzaldehyde and acetaldehyde were diluted to 1000 ppm. For Dowicil 200, Glydant and Glydant XL-1000, and Germall 115 preservatives, 0.4 g of each were dissolved in 40 mL 85:15 water:MeOH solution to yield a 1.0% solution of each. For Steol CS-370, Stepanol WAT-K, and Stepanol AM surfactants, 0.4 g of each were dissolved in 40 mL 85:15 water:MeOH solution to yield a 1.0% solution of each. For Steol CS-270 and Stepanol WAT surfactants, 0.8 g of each were dissolved in 40 mL 85:15 water:MeOH solution to yield a 2.0% solution of each.
References
- Steinberg, D.C. Preservatives for Cosmetics. Allured Publishing Corp.: Carol Stream, IL, 2006; pp 14.
- Michels, J.J. Improved measurement of formaldehyde in water-soluble polymers by high performance liquid chromatography coupled with post-column reaction detection. J. Chromatogr. A 2001, 914, 123-9.
The authors are with Aura Industries, Inc., 545 Eighth Ave., New York, NY 10018, U.S.A.; tel.: 212-290-9190; fax: 212-290-9191; e-mail: [email protected].