Perchlorate is both a naturally occurring chemical and a man-made inorganic ion. Naturally occurring perchlorate is a contaminant in fertilizers; man-made perchlorate is used in applications such as tanning and leather finishing, rubber manufacture, paint and enamel production, additives in lubricating oils, and as a primary ingredient of solid rocket propellant.
Due to improper usage and disposal, highly water-soluble perchlorate migrates through aquifers and surface water, contaminating soil, drinking water, and irrigation water.
Thirty-five states have detected perchlorate in drinking water at levels higher than expected. This water is also used for crop irrigation; as a result, there is concern about the food safety of fruits, vegetables, and grain, as well as their use as animal feed in the southwestern U.S.
Today, Maryland, Massachusetts, and New Mexico have established a 1-ppb perchlorate action limit, and California and Texas have established a 4-ppb action limit; action limits are higher in Nevada and Arizona. In February 2005, the U.S. EPA established an official perchlorate reference dose (RfD) of 0.0007 mg/kg/day. However, sub-ppb detection limits are desirable.
Experimental
U.S. EPA Method 314.1, "The Determination of Perchlorate in Drinking Water Using Ion Chromatography” (2004), is the current approved method for perchlorate in drinking water. This ion chromatography method is capable of detecting sub-ppb ClO4, but is limited by sample ionic strength, mainly chloride and sulfate concentrations, adversely limiting detection. Other common anions (thiosulfate, thiocyanate, and iodide) elute in the same region; for analyte identification, a simpler, alternative method is required. The key to this application is the resolution of <0.5 ppb perchlorate from mid-ppm levels of chloride and sulfate. Sulfate is the main interferent with Method 314.1, and the 34S isotope as H34SO–4, at 4.2% abundance, has the same MS-MS transition as perchlorate. It is critical to remove SO4 or provide baseline resolution from perchlorate.
By combining chromatographic selectivity and MS selectivity and sensitivity, perchlorate can be determined in high total dissolved solids (HTDS) water at the sub-ppb levels. HTDS is a synthetic solution defined as 1000 mg/L each of bicarbonate, chloride, and sulfate prepared in drinking water.

Figure 1 - One part per billion perchlorate in HTDS.
Using liquid chromatography principles, increased concentrations of organic solvent (acetonitrile [ACN] in this case because of low backpressure), perchlorate can be chromatographically positioned after high chloride and before high sulfate. Use of ammonium bicarbonate eluent allows for the sub-ppb determination of perchlorate within 15 min without the need for sample preparation (Figure 1).
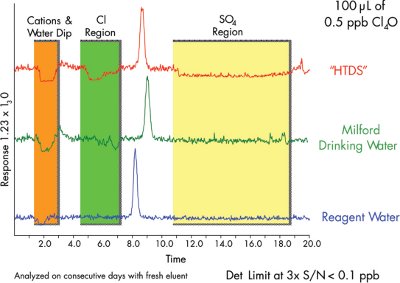
Figure 2 - Perchlorate detection (0.5 ppb) in three different sample matrices by LC-MS-MS.
Ammonium bicarbonate is also a volatile buffer conducive to use with MS. Any sodium (Na) or potassium (K) based eluents require chemical suppression prior to MS. Chemical suppression converts KOH to H2O. Chemical suppression is not needed for this application.
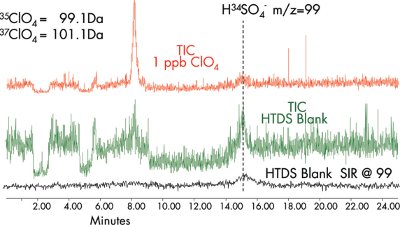
Figure 3 - Sulfate interference.
Quantitative measurement of sub-ppb perchlorate requires a method detection limit (MDL) of less than 0.5 ppb perchlorate, using either 3× signal-to-noise ratio (S/N) or U.S. EPA protocols. Figure 2 shows a 0.5-ppb perchlorate standard prepared in reagent water, drinking water, and HTDS solutions. The limit of detection (LOD) is approx.<0.1 ppb, defined as 3× S/N, and the limit of quantification (LOQ) is 0.2 ppb, at 10× S/N. This LC-MS-MS method meets the requirements. Lower detection may be achieved by using a larger injection volume.

Figure 4 - Perchlorate linearity.
Multiple reaction monitoring (MRM) MS is used to monitor the transition of perchlorate to chlorate, 99.1 82.7 (loss of 1 oxygen), for perchlorate quantification. Positive perchlorate confirmation is achieved using a second transition of 101.1 > 84.7 for the 37Cl isotope and the ion ratio of the 35Cl and 37Cl isotopes. Although H34SO–4 sulfate may give a 99 > 83 response, it will not yield the required Cl isotope ratio (Figure 3).
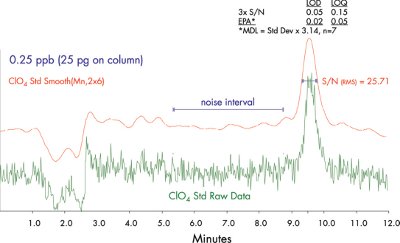
Figure 5 - Perchlorate LOD and LOQ after chromatographic smoothing.
The linearity of this method was evaluated between 0.25 and 100 ppb perchlorate containing 10 ppb internal standard using a 100-μL injection. The calibration curve, using 1/× weighting (Figure 4), shows good linearity between 0.25 and 10 ppb. Similar data are observed with calibration standards prepared in HTDS solution and drinking water. Figure 5 shows the LOD and LOQ for perchlorate quantification (LOD = 0.02 ppb and LOQ = 0.05 ppb by the U.S. EPA definition of MDL).
Conclusion
This work demonstrates the feasibility of using the IC-Pak™ Anion/HR (A/HR) chemistry and the Quattro micro™ API mass spectrometer (both from Waters Corp., Milford, MA) for the selective analysis of perchlorate in environmental waters. Chromatographic selectivity of the polymethacrylate-based IC-Pak A/HR column using ammonium bicarbonate and ACN eliminates the interference from sulfate and the need for sample preparation to remove chloride and sulfate.
LC-MS-MS is capable of detecting <100 ppt in HTDS water, such as 0.3% brackish water or soils leachate, within 15 min injection-to-injection. This method has also been evaluated for soils and industrial sludge.
The 50% ACN eliminates any buildup of neutral total organic carbon (TOC) affecting column performance. This TOC effect is normally observed with all aqueous eluents.
This chromatographic method has not been fully validated and represents feasibility. It is the responsibility of the user of this method to provide an initial demonstration of performance on the expected matrices before quantitative work can begin. However, U.S. EPA Method 331.0 (2005) has validated the MS-MS acquisition and processing for perchlorate in drinking water and wastewater using a similar column and eluent.
Mr. Romano is Senior Marketing Manager, Waters Corp., 34 Maple St., Milford, MA 01757, U.S.A.; tel.: 508-482-2963; fax: 508-482-3085; e-mail: [email protected].