The analysis of a sample generally involves a series of steps in which glassware and other sample processing hardware are used. In the present market, sample preparation equipment, accessories, and supplies are produced in a wide variety of materials to fulfill experimental requirements. For example, the use of amber glass containers can prevent photochemical changes, and it has been reported that some coloring metals can leach out and complex the compounds such as organic acids/amines and cyanide, possibly leading to the loss of targeted analytes over time during storage. Therefore, incorrect use of analytical hardware may violate the validation of analytical methods as regulated by the guidelines from institutions such as the U.S. Environmental Protection Agency (EPA), the U.S. Food and Drug Administration (FDA), and the International Conference on Harmonization (ICH). Overall, by running method blanks under the conditions of the analysis, all of the materials and reagents must be demonstrated to be free from interferences.
In the analytical laboratory, syringe needles are commonly inserted, through open-top caps fitted with septa lined with polytetrafluoroethylene (PTFE), into containers such as vials and volumetric flasks for sampling or transferring stock solution. This is even more necessary during the automation of analytical methods, in which liquids such as standard and derivatization reagent need to be added before sampling. When the needle pierces the septum, the rubber is exposed to the sample by either headspace or direct contact, which will affect the quality of the data in various ways. This application note discusses the influences of pierced PTFE/silicone septa in GC-MS experiments. Two examples are given: the loss of nonpolar volatile organic compounds (VOCs) in aqueous media and the contamination of organic solvent sample matrix.
Experimental
The experiment was carried out on a Saturn 3800 GC/2000 ITMS system (Varian, Walnut Creek, CA) fitted with an Rtx-5MS fused-silica column (30 m × 0.25 mm × 0.25 μm film) (Restek, Bellefonte, PA). Combi-Pal autosamplers (Leap Technologies, Carrboro, NC) were installed for both solid-phase microextraction (SPME) and liquid injection. Polydimethylsiloxane (PDMS, 100 μm) SPME fiber with a 23-gauge protective needle (Supelco, Bellefonte, PA) and a 10-μL syringe with fixed cone tip needle (SGE International Pty. Ltd., Victoria, Australia) were used, respectively. Splitless injection mode was applied with 1-min desorption for SPME and an aliquot of 1-μL sample in the liquid injection experiment. The injector was set at a temperature of 250 °C, and helium was used as the carrier gas at a flow rate of 1 mL/min. Details of the instrument setup can be found elsewhere.1
Loss of nonpolar VOCs
Figure 1 - Progressive loss of BTEX after spiking using a syringe by puncturing the PTFE/silicon septa.
Benzene, toluene, ethylbenzene, and o-xylene (collectively referred to as BTEX) were used, which represent a group of volatile nonpolar organic compounds. In the analysis of trace VOCs in soil and residual solvent in drug tablets, suspended in aqueous media, a progressive loss of BTEX was noted after spiking via septa. This was initially considered to be caused by some type of binding or by poor sealing, as discussed elsewhere for the use of vials, septa, and the two combined.2 To explore the origin of the loss, a set of 3-mL aliquots of pure water sealed in 10-mL headspace vials with crimp-cap closures (MicroLiter Analytical Supplies Inc., Suwanee, GA) were fortified to 30 μg/L in each component of BTEX by spiking 10 μL of stock methanolic solution into each vial during the same time period. They were sampled by SPME after a spike residence time of 0 (sampled immediately after spiking) to 25 hr (Figure 1). Each data point is an average of two replicates analyzed consecutively; selected ions (m/z 78, benzene; m/z 91, toluene; m/z 106, ethylbenzene and oxylene) were used to calculate the response (area count). Ten-minute SPME sampling by headspace was used, during which equilibrium was reached for all four compounds.1 The following equation shows that the amount of analyte absorbed by the coating at equilibrium, nf, is linearly proportional to the initial concentration in the sample, C0:
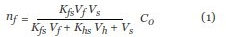
where Kfs and Khs are the fiber/sample and headspace/sample distribution coefficient and Vf, Vs, and Vh are the volume of the fiber coating, the sample, and the headspace. The sampling amount (represented by the area count) of ethylbenzene and oxylene is higher than that of benzene and toluene because of their higher partition in PDMS fiber. Figure 1 shows that the level of BTEX exhibited an exponential mode of decrease. Furthermore, the loss of ethylbenzene and o-xylene is significant (84 and 79% in 25 hr, respectively) and much higher than that of benzene and toluene (47 and 68% in 25 hr, respectively). An alternate set of data acquired with screw-cap closures demonstrated the same rate of constituent loss. The behavior thereof suggests that the seal failure is caused mainly by partition into exposed rubber, not by a leak in the contacting area between the septa and glass rim, which would lead to a more considerable loss for higher-volatility benzene and toluene. In order to assess the effect of leakage in the joint area, the BTEX methanolic solution was spiked under water immediately before the vials were capped. For the vials with both the crimp-cap and screw-cap closures, no loss was observed for four compounds in 15 hr. It should be noted that the loss rate of BTEX is strongly associated with the matrix properties, which affect the partition of analytes into headspace and pierced septa.3–5
Figure 2 - Calibration curves of BTEX.
Understanding the potential analyte loss, and its possible effect on data quality, is very important in designing the experimental procedure. When all calibration standards were first prepared by consecutively spiking the BTEX methanolic solution via septa into 10-mL vials containing 3 mL of water (then starting the analysis in sequence from low to high concentration to construct the calibration curve), the dynamic process in the loss of BTEX impaired a series of method validation parameters, such as precision and accuracy, linearity and range, and method detection limits (MDLs). This was particularly true when the calibration time was long and for xylenes and ethylbenzene. Even though the calibration curve was still linear, the accuracy (indicated as recovery) could be poor because of the deviated response factor caused by the loss of analytes. Recognizing the influence of pierced silicone septa, the author prepared eight level calibration standards of 0.09, 0.73, 2.18, 7.27, 14.53, 29.07, 43.60, and 72.67 μg/L immediately prior to sampling. Figure 2 presents calibration curves showing improved validation parameters such as linearity and range, especially for o-xylene and ethylbenzene, in which the calibration was completed in 250 min with each standard analyzed in duplicate. In general, it is recommended that sampling of volatile analytes in aqueous matrix be conducted immediately after the liquid is added using a syringe via septum.4 Alternatively, the addition can be accomplished manually under the sample matrix immediately before the vials are capped.
Contamination
Figure 3 - Typical mass chromatogram of volatile residue in hexane caused by PTFE/silicone septa: a) hexane extract of a piece of septum; b) vial septum was punctured once by a syringe to sample 1 μL hexane for GC analysis; c) hexane in the last vial was reanalyzed after 3 days; and d) analyzed immediately after 100 μL hexane was added from one vial to another (the septum was punctured 10 times to transfer the liquid using the 10-μL syringe). The response scale in b, c, and d was amplified 30 times for clarity.
In the GC-MS analysis by liquid injection, the autosampler was used for solvent addition, standardization, and calibration. Contamination from the silicone septa was identified, which is related to cyclic PDMS residue and curing agents,6 in which hexa-methylcyclotrisiloxane is designated as D3, octamethylcyclotetrasiloxane as D4, decamethylcyclopentasiloxane as D5, and so forth. The 350-μL target microsampling vials with target 10-mm screw cap (National Scientific Co., Duluth, GA) were examined in this section. Figure 3a shows the chromatogram of the extract of a piece of septum, which presents a series of peaks centered at D7.
One possibility of silicone septa contamination relates to diffusion through the headspace, as characterized in Figure 3b and c, where the septum was punctured once by the 10-μL syringe needle with a 250-μL aliquot of hexane sealed in the vial. With the sensitivity of the method herein, no contamination was detected 8 hr after puncturing, and the volatile residue accumulated in hexane over a long period of time, with the peaks centering at D5 due to the volatilization effects. In this process, the organic solvent acts as a sorbent to enrich the volatile residue through the headspace. Also noted was the transfer of a tiny piece of septum into solvents, standards, and sample extracts by the syringe needle used for injection, presenting a randomly instantaneous impact of interferences (Figure 3d), even though this was minimized by selection of the correct needle design.
The contaminants from septa may sometimes affect the assay, especially the determination of analytes in very low concentration,7,8 and the study of biological samples (e.g., metabolite profiling, in which hundreds of peaks are detected to be identified and quantified). In the author’s laboratory, a blank run is performed regularly to monitor contamination, and a schedule is set up to clean accumulated contaminants in the vials for solvents and standards. Based on routine operation, several opportunities exist to prevent the interference of septa contamination. It is critical to minimize the exposure of the organic solvent matrix to the pierced PTFE/silicone septa before sampling and liquid transfer. When necessary, the PTFE/silicone septa can be processed by heat treatment under vacuum, which can considerably decrease the level of volatile chemicals in septa.7 PTFE liner, which is inert but shows inferior vapor sealing ability, can be used to replace the PTFE/silicone septum before the test in cases where the inferior sealability has little effect on sample composition. It was noted that similar interference can be caused by other sources such as GC injector septa and column stationary phase.8
Conclusion
Interference is one of the most challenging problems found in analytical method validation. The criteria discussed here to prevent silicon septa interference can be extended to other septa, including butyl rubber and chlorobutyl rubber, as well as to other experiments.
References
- Wang, Y.; O’Reilly, J.; Chen, Y.; Pawliszyn, J. Equilibrium in-fibre standardisation technique for solid-phase microextraction. J. Chromatogr. A 2005, 1072, 13–17.
- Baltensperger, B. Vials, caps, septa & various products in comparison. Lab Asia 2004, 11, 12–14.
- Technical guide 59895A: a technical guide for static headspace analysis using GC. Restek Corp., Bellefonte, PA, 2000, http://www.restek.com/pdfs/59895A.pdf.
- Method 5035A: Closed-System Purge-and-Trap and Extraction for Volatile Organics in Soil and Waste Samples. U.S. Environmental Protection Agency, 2002, www.epa.gov/epaoswer/hazwaste/test/pdfs/5035a_r1.pdf.
- Lin, Y.S.; Smith, T.J.; Wypij, D.; Kelsey, K.T.; Sacks, F.M. Association of the blood/air partition coefficient of 1,3-butadiene with blood lipids and albumin. Environ. Health Perspect. 2002, 110, 165–8.
- Homma, H.; Kuroyagi, T.; Izumi, K.; Mirley, C.L.; Ronzello, J.; Boggs, S.A. Evaluation of surface degradation of silicone rubber using gas chromatography/mass spectroscopy. IEEE Transact. Power Del. 2000, 15, 796–803.
- Chambers, D.M.; McElprang, D.O.; Mauldin, J.P.; Hughes, T.M.; Blount, B.C. Identification and elimination of polysiloxane curing agent interference encountered in the quantification of low-picogram per milliliter methyl tert-butyl ether in blood by solid-phase microextraction headspace analysis. Anal. Chem. 2005, 77, 2912–19.
- De Zeeuw, J. How to minimize septum problems in GC. Am. Lab. 2005, 37(19), 18–19.
Dr. Wang is a Research Chemist for Metabolites Analysis, McMaster University, Biology Dept., 1280 Main St., W., Hamilton, Ontario, L8S 4M1, Canada; tel.: 905-523-1489; e-mail: [email protected].