Some types of electronic noses use ultrafast gas chromatography to measure and capture a unique signature of products via a chemical or olfactive fingerprint. The HERACLES Electronic Nose (Alpha M.O.S., Toulouse, France) can be used for laboratory measurements and, in a portable version, for on-field analysis. It allows liquid, gas, or solid sample analysis with several injection modes (headspace, solid-phase microextraction [SPME], and trap).
The instrument integrates two short columns of different polarity placed in parallel, associated with two flame ionization detectors (FIDs), permitting two chromatograms to be generated simultaneously. A typical analysis can be run in 15–120 sec. For example, volatile organic compound (VOC) analysis is performed in less than 60 sec, with a sensitivity reaching ppb concentrations, due to an integrated trap using an absorbent support.
Another feature of the instrument is its ability to perform complete data treatment, with classical GC functionalities and E-Nose olfactive fingerprint software: radar plot, principal component analysis (PCA) to compare olfactive profiles, partial least squares (PLS) model to quantify concentrations or scores, and quality control cards (SQC) to monitor conformity checking.
This application note presents performance of the instrument using two applications: the analysis of current volatile and semivolatile compounds, and the quality control of flavored mouthwash solutions and quantification of menthol in these products.
Analysis of volatile and semivolatile compounds

Figure 1 - HERACLES system.
In order to perform rapid analysis and identification of current volatile and semivolatile compounds, specific chromatographic and integration methods were developed with the HERACLES Electronic Nose. The short columns (1–3 m long) allow a chromatogram to be run in seconds, whereas classical gas chromatography can take 45 min or more. The presence of two columns of different polarity (one nonpolar and one slightly polar) in parallel (Figure 1) provides dual information and thus ensures reliable identification of chemical compounds.
Samples and operating conditions
Fourteen current volatile and semivolatile compounds were analyzed with the HERACLES Electronic Nose: isopentane, acetonitrile, acrolein, pentane, propanol, acrylonitrile, vinyl acetate, isobutanol, n-butanol, benzene, cyclohexane, mercaptoethanol, chlorobenzene, and ethylbenzene. The samples were injected in manual headspace mode; 250 μL of neat compound was introduced in 20-mL headspace vials. The vials were then sealed and stabilized at 20 ± 2 °C for one night.
Table 1 - Retention times of the 14 compounds on the columns

The saturated vapors were then diluted according to the volatility of the compounds to avoid column overloading. Each compound was individually injected using a 20-mL hot gastight syringe.
Results
An integration method including peak tables and integration events was built. The retention times are listed in Table 1. It can be seen that all compounds were detected and analyzed in less than 25 sec. When strong coelution occurred, groups of peaks were considered for identification. For instance, Figure 2 shows that ethylbenzene and chlorobenzene are resolved on column 1 but not on column 2. Thus, all compounds could be identified due to unique retention times on the two columns; all compounds coeluting on a column were separated on the second one.
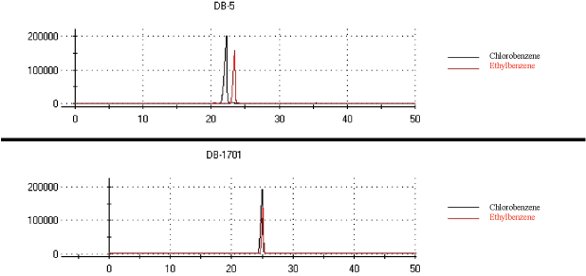
Figure 2 - Chromatograms of chlorobenzene and ethylbenzene using the columns.
Personal-care products: Quality control of mouthwash
Flavor is an important feature of a mouthwash since it is the most perceptible parameter. Spice flavors such as peppermint, menthol, methyl salicylate, and eugenol are generally added to mouthwash formulas to improve taste and acceptability. To guarantee reproducible taste and flavor, robust and simple quality control methods are crucial. The HERACLES Electronic Nose was used to analyze various mouthwash products in order to develop a QC method based on the different flavor profiles, then to determine the concentration of menthol in mouthwash solutions.
Samples and operating conditions
Table 2 - Analytical conditions for the analysis of mouthwash

In the first step, a reference mouthwash representing good quality (as defined by the manufacturer based on human assessment) was analyzed with the HERACLES Electronic Nose in order to build a quality control model. Next, blind samples were analyzed in order to assess their quality and whether to accept or reject products. In a second step, seven solutions containing various amounts of menthol were analyzed. The objective was to set up a calibration model that allowed the quantification of menthol in new samples. Analytical conditions are shown in Table 2.
Results
1. Quality control model. After analyzing all mouthwash samples with the HERACLES Electronic Nose, significant peaks in the reference samples were selected in order to set up a quality control chart (Figure 3). The gray band defines the limits of acceptable quality. Unknown samples (in green) that fall outside this conformity band are identified as out of specification.

Figure 3 - Quality control chart for mouthwash analysis—visualization of chromatogram differences on a pie/bar chart.
With HERACLES software, it is possible to visually point out differences between chromatographic profiles. The off-specification sample chromatogram can be easily compared to the chromatogram library of all good samples. A pie chart (Figure 3) indicates the percentage of difference: In this case, 25% of the peaks are different (in green) and 75% are similar (in blue). The bar-code chart (Figure 3) allows one to identify the peaks that explain the differences: Here the difference is mostly due to light VOCs and the heaviest compounds (green bars).
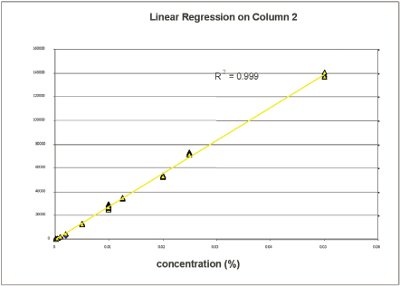
Figure 4 - Partial least squares model.
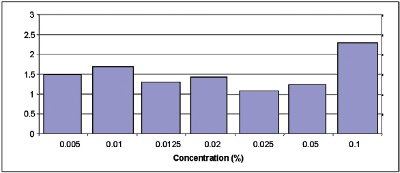
Figure 5 - RSD by sample.
2. Quantification of menthol in mouthwash. The calibration model in Figure 4 shows an important linearity: R2 = 0.999 from 0.005% to 0.1% w/v, and a very low limit of detection of menthol: 0.1 ppm in liquid phase. Thus, the instrument permits the reliable quantification of compounds such as menthol at low concentrations in personal-care products.
Therefore, with an RSD of measurements below 5% (Figure 5), the HERACLES Electronic Nose offers analytical solutions to:
- Perform fast and accurate quality control of the global flavor profile
- Guarantee the continuous rapid monitoring throughout the addition of specific compounds in order to reach the desired concentrations of a compound when formulation blends are being prepared
- Monitor batch-to-batch consistency of personal-care products, with the identification of compounds responsible for defaults.
Mrs. Bonnefille is Communication Manager, Alpha M.O.S., 20 ave. Didier Daurat, 31400 Toulouse, France; tel.: +33 5 62 47 64 55; fax: +33 5 61 54 56 15; e-mail: [email protected].