Quantum dots are semiconductor
nanocrystals that have
size-dependent properties,
unlike bulk materials, as indicated
by the well-studied
CdSe system.1 They are characterized
by a band gap, and
corresponding to the band
gap there is an absorption in
the UV-VIS region and an emission in the visible region
followed by excitation in the
band gap. It is well established
that the emission spectrum
is sensitive to trace levels
of dopants such as Mn2+,
as has recently been demonstrated
for ZnS quantum
dots.2 The goal of the authors’
research program is to
achieve a fundamental understanding
of the synthesis and spectroscopic properties of
quantum dots other than the
CdSe system.
The authors conducted a number of
studies with the ZnS quantum dots.
One of their research objectives is to
be able to dope the quantum dots
with magnetic metal ions such as
Mn2+ to impart to them interesting
and useful spectroscopic and magnetic
properties that could lead to
practical applications. In this regard,
the authors are investigating quantum
dots that can be doped with
trivalent lanthanide metal ions,
which may lead to doped materials
with valuable spectroscopic and magnetic
properties. The systems of
interest for doping with lanthanide
metal ions are the PbS and CdS
quantum dots, in which the ionic
radii of Pb2+ and Cd2+ are close to
those of trivalent lanthanides. Doping
these quantum dots with trivalent
lanthanide ions is an ongoing
project in the authors’ laboratory and
these results will be published separately. In contrast, in the ZnS quantum
dot lattice, the ionic radius of
Zn2+ is much smaller than those of
the trivalent lanthanides.
This paper discusses preliminary studies
on the synthesis and characterization of
PbS quantum dots that are doped and
undoped with Mn2+. To the authors’
knowledge, the synthetic procedure
reported here is the first to employ an
aqueous phase synthesis at ambient
temperatures to obtain undoped and
doped PbS quantum dots. PbS quantum
dots, reported in the literature, are in
thin film and other matrices and in
nonaqueous solvents.3–14
Materials and methods
All compounds employed in the synthesis
were acquired from Aldrich
Chemical Co. (Milwaukee, WI) and
were used without further purification.
A typical synthesis of PbS quantum
dots doped with Mn2+ is described here, and the nanocrystals
without Mn2+ were synthesized
using the same procedure.
The stabilizer sodium
polyphosphate Na(PO3)n
(10.2 g) was dissolved in 70
mL of MilliQ water (Millipore
Corp., Billerica, MA). A
solution of 3.31 g Pb(NO3)2
in 10 mL of MilliQ water was
added to the stabilizer and stirred at room temperature
for 90 min. Following the
addition of Pb2+, a white precipitate
formed, indicating a
complex formation between
the Pb2+ and (PO3)n. After 90
min, the solution was filtered,
after which 1 mL of 1.80 g
Mn(NO3)2 dissolved in 10 mL
of MilliQ water was added to
it, corresponding to 10% doping
with Mn2+. This was
immediately followed by the
dropwise addition of a 10-mL
solution of 2.40 g Na2S in
MilliQ water. The mixture was stirred
at room temperature for 1 hr. The solution
was centrifuged for 10 min and
the supernatant was decanted. The
slurry of black Mn2+-doped PbS quantum
dots was washed twice with
MilliQ water. The first wash was discarded
and the second was saved. The
second wash was used to examine the
UV-VIS absorbance, emission, and transmission electron microscopy (TEM) image of the quantum dots.
The remaining slurry was dried and
used for electron paramagnetic resonance (EPR) analysis.
The UV-VIS spectra were obtained
with a Lambda 20 spectrophotometer
and the emission spectra were recorded
with an LS 50B spectrofluorimeter
(both from PerkinElmer, Shelton,
CT). The EPR spectra of the solid PbS
samples were recorded at room temperature
with a JEOL JES-TE100 (JEOL-USA,
Peabody, MA) at a microwave frequency of 9.0559 GHz over a sweep
width of 300 mT (T = tesla).
Results and discussion

Figure 1 - TEM image of PbS quantum dots, indicating an average
size of 100 nm and nanoparticles that are fused.
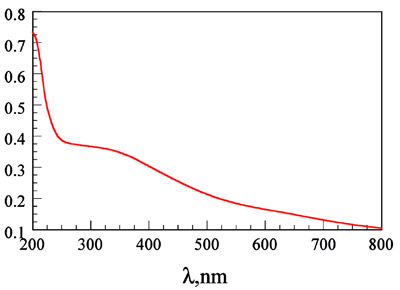
Figure 2 - UV-VIS spectrum of a dispersion of PbS in water, exhibiting
a shoulder at 350 nm corresponding to the band gap of the nanocrystals.
The TEM image of the PbS quantum
dots doped with Mn2+ is displayed in
Figure 1. The extent of doping is estimated
to be about 1% from prior studies
with ZnS quantum dots, even though
the concentration of Mn2+ in the synthesis
is 10% of the Zn2+ concentration.2
The average size is about 100 nm, with
nanoparticles that are both smaller and
continued
larger than this size (see Figure 1). In
comparison, ZnS yielded nanoparticles
that were 5 nm on average under the
same conditions.2 The UV-VIS spectrum
of a dispersion of PbS (the supernatant
from the wash of the nanocrystals)
is shown in Figure 2, with a shoulder at 350 nm. This corresponds to the band
gap of the PbS semiconductor nanocrystals;
it is similar to those reported in the
literature5–8 and is at a lower energy than
the ZnS quantum dots, which have a
shoulder at 300 nm. When this solution
was examined for emission by scanning
the excitation and emission spectra, no
measurable emission could be observed.
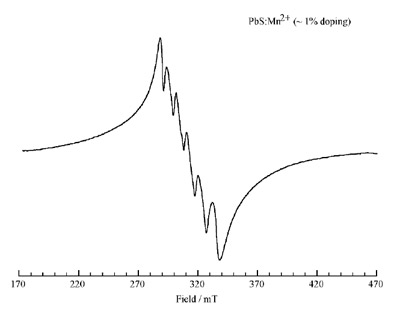
Figure 3 - EPR spectrum of the Mn2+-doped PbS quantum dots,
indicating the expected six lines. Doping occurs predominantly on the
surface of the nanocrystals.
Extended spectral accumulations and
signal averaging by excitation at 350
nm corresponding to the band gap
also yielded no measurable emission
from 400 to 900 nm. This is in agreement
with previous observations in
the literature, where the emission of
PbS in the aqueous phase is quenched
by water and can be observed only in
nonaqueous media with PbS
nanoparticles coated with an organic
compound such as thiol or in thin
films.6–8,10,12,13 The EPR of the PbS
quantum dots doped with Mn2+ is
shown in Figure 3.
To the authors’ knowledge, this
paper is the first to report on the
EPR of PbS nanoparticles doped
with magnetic nuclei. The EPR
demonstrates the expected six peaks
for Mn2+, which are not well separated.
This pattern, as shown in the
case of ZnS quantum dots doped
with Mn2+, indicates that in the
case of PbS quantum dots the ions
are present predominantly on the
surface of the nanocrystals. As a
result, extensive interaction occurs
between the Mn2+ ions, yielding an
unresolved six-line pattern.
Conclusion
PbS quantum dots of 100 nm average
size doped with Mn2+ were prepared in
aqueous phase at room temperature
using polyphosphate as the stabilizer.
The doping of the Mn2+ ions was
shown to occur predominantly on the
surface of the nanocrystals by EPR.
References
- Bandyopadhyay S, Nalwa HS, eds.
Quantum dots and nanowires, 1st ed.
Stevenson Ranch, CA: American Scientific
Publishers, 2003.
- Beerman PG, McGarvey BR, Muralidharan
S, Sung R. EPR spectra of Mn2+-
doped ZnS quantum dots. Chem of
Mater 2004; 16:915–18.
- Zheng Z, Wang S, Yang S. Synthesis and
characterization of PbS nanocrystallites
in random polymer inomer. Chem of
Mater 1999; 11:3365–9.
- Zhu J, Liu S, Plachik O, Koltypin Y,
Gedanken A. A novel sonochemical
method for the preparation of nanophasic
sulfides: synthesis of HgS and PbS
nanoparticles. J Solid State Chem 2000;
153:342–8.
- Patel AA, Wu F, Zhang JZ, et al. Synthesis,
optical spectroscopy and ultrafast
electron dynamics of PbS nanoparticles
with different surface capping. J Phys
Chem B 2000; 104:11,598–605.
- Chen S, Traux LA, Sommers JM.
Alkanethiolate-protected PbS nanoclusters:
synthesis, spectroscopic and electrochemical
studies. Chem of Mater 2000;
12:3864–70.
- Yang P, Song CF, Liku MK, et al. The
luminescence of PbS nanoparticles
embedded in sol-gel-silica glass. Chem
Phys Lett 2001; 345:429–34.
- Kim D, Mishima T, Tereatani N,
Mizoguchi K, Nakayama M. Visible
luminescence from PbS quantum dots
prepared by colloidal method. Proceedings,
International Conference
on Narrow Gap Semiconductors and
Related Small Energy Phenomena,
Physics, and Applications: Institute
of Pure and Applied Physics Conference Series 2, Ishikawa, Japan,
2001:178–80.
- Jiang P, Liu ZF, Cai SF. Growing
monodispersed PbS nanoparticles on self-assembled
monolayers of 11-mercapto-undecanoic
acid on Au(111) substrate.
Langmuir 2002; 18:4495–9.
- Flores-Acosta M, Sotelo-Lerma M,
Arizpe-Chavez H, Castillon-Barraza
FF, Ramierz-Bon R. Excitonic absorption
of spherical PbS nanoparticles in
zeolite A. Solid State Commun 2003;
128:407–11.
- Wang D, Yu D, Mo M, Liu X, Qian Y.
Hydrothermal preparation of one-dimensional
assemblies of PbS nanoparticles. Solid State
Commun 2003; 125:475–9.
- Xiang J, Yu SH, Liu B, Xu Y, Gen X,
Ren L. Shape controlled synthesis of PbS
nanocrystals by a solvothermal-microemulsion
approach. Inorg Chem
Commun 2004; 7:572–5.
- Zhang B, Li G, Zhang J, Zhang Y, Zhang
L. Synthesis and characterization of PbS
nanocrystals in water/C12E9/cyclohexane
microemulsions. Nanotechnology 2003;
14:443–6.
- Joshi RK, Kasnjilal A, Sehgal HK. Solution
grown PbS nanoparticle films. Appl
Surf Sci 2004; 221:43–7.
The authors are with the Department of Chemistry
and the Nanotechnology Research and Computation
Center (NRCC), Western Michigan
University, Kalamazoo, MI 49008, U.S.A.;
tel.: 269-387-3656; fax: 269-387-2909;
e-mail: [email protected]. Support for
this research from the W.M. Keck Foundation
(Los Angeles, CA) is gratefully acknowledged.