The measurement of chlorophyll fluorescent
signals from photosynthetic
enzyme complexes (PEC) has become
one of the most powerful indicators for
ecophysiologists. PECs have been used
for more than three decades in
research laboratories to determine the
toxicological impact of a vast array of
chemicals.1–11 Photosynthesis is the
absorption of light energy and its conversion
into stable chemical potential
in chloroplasts. The major part of the
absorbed light energy is used to drive
photochemical reactions (redox complexes
in an electron transport chain).
However, a part of this absorbed light
energy is emitted in the form of fluorescence
and nonradiative energy dissipation.
The presence of electron
transport inhibitors (see Figure 1 for
inhibition sites) can modify the balance
between those energetic processes
promoting the dissipation process. Based on
chlorophyll fluorescence emission by PECs, the toxicity
of a sample is indicated by the modification of fluorescence
parameters. This plant bioassay was seen to rapidly
evaluate the toxicity of an effluent, thus proving the efficacy
of the treatment.
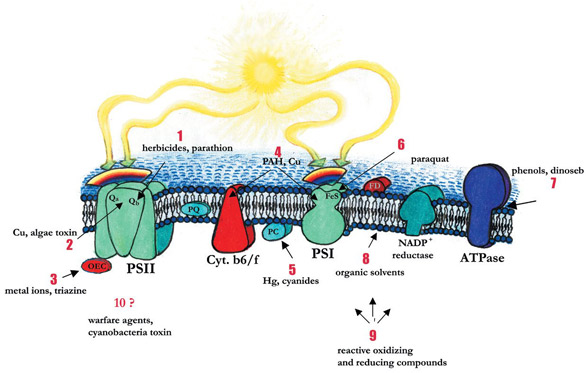
Figure 1 - Representation of PEC with inhibition sites.
This widely accepted technique is the basis of an instrument
and consumables, the LuminoTox (Lab-Bell Inc., Québec,
Canada).12 The test kit is supplied in two parts: 1) a
portable, robust, and compact instrument, and 2) the consumables,
the PECs. The PECs have been stabilized following
a proprietary technique to ensure a commercially acceptable
shelf life. The test takes only 15 min to perform,
allowing rapid screening of samples to be selected for further
chemical and/or bioassay testing. The system is automated in
order to provide on-line monitoring of accidental or intentional
toxic discharges in the water supply or other environmental infrastructure.
This paper presents the principle supporting the LuminoTox
rapid toxicity screening device. It proposes a simple yet
effective method for selecting positive samples as opposed to
time-consuming and expensive chemical and/or bioassay
testing methods. Results obtained from effluents before and
after treatment of industrial wastewater plants (pulp and
paper mills and mining industries), municipal wastewater
plants, and landfill sites are presented. They demonstrate the
system’s ability to assess the efficacy of treatment.
Principle of operation
The LuminoTox measurement uses a fluorescence dissipation
process emitted by PSII complexes. The variable fluorescence
of chlorophyll a is considered an indicator of electron transport
efficiency since it is mostly related to the redox state of
the PSII electron acceptor plastoquinone A (QA).5,10
To evaluate the photochemical quantum yield (ϕP)—also
called photosynthetic efficiency—by fluorescence measurements,
the PECs need to be dark adapted. Two levels of fluorescence
are required. The first level (F1) is obtained after the
application of a low-intensity light in order to determine the
fluorescence of chlorophyll molecules that absorb light in PEC.
If this light intensity is too weak to drive the photosynthesis process, the entire PECs will be oxidized or in an “open state.”
The second level (F2) is reached following the application of a
high-intensity light pulse. The saturating pulse will induce the
closure of all enzyme complexes (reduced state).
In the LuminoTox, light intensities have been chosen to provide
partial oxidation and reduction of PEC. The photochemical
quantum yield (ϕP) is evaluated as follows:3,4

In the LuminoTox, the relative photochemical quantum
yield, named the relative efficiency (eff), is evaluated in
order to obtain better sensitivity.
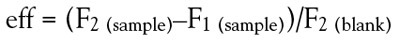
F2 (blank) is referred to as the fluorescence value obtained in
the control solution.
Materials and method
Apparatus
LuminoTox dedicated fluorometers were used. The test
does not work with conventional fluorometers, but specialized
fluorescence photosynthetic measurement apparatus
may be used. No calibration is required. The LuminoTox
apparatus is calibrated upon manufacture.
Reagents
The following reagents were employed:
- PEC–photosynthetic enzyme complexes
- Reaction buffer
- Organic standards, atrazine in water (three concentrations)
- Inorganic standards, copper in water (three concentrations).
Preparation of PEC
The reaction buffer was added to the PEC and mixed vigorously
until all the complexes were totally solubilized. A
period of 15 min had to elapse before using the enzymes.
The solution was mixed occasionally during the waiting
time. It is important to agitate before each measurement.
Procedure

Figure 2 - Test procedure.
A user-friendly method has been developed that can be easily
deployed in the field (Lab-Bell). The procedure requires
only standard laboratory supplies such as disposable cuvettes
and syringes (Figure 2).
Results and discussion
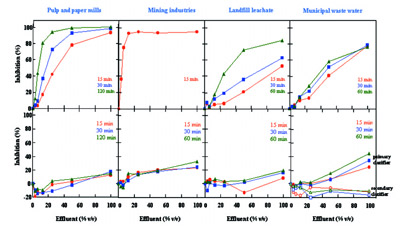
Figure 3 - Results obtained from wastewater samples before and after
treatment.
Toxicity control is a major driving force in the follow-up of
urban wastewater treatment directives. Most biological
treatment plants that accept industrial wastewater experience
problems from time to time with toxic substances.
This problem is usually due to the presence of toxic chemicals
in the incoming waste stream. A fast, reliable test was
needed to measure the toxicity of industrial wastewater and
to evaluate the efficiency of the treatment. The LuminoTox is not only useful for urban wastewater treatment
plants, but also has the potential to help industries eliminate
chemicals used or produced. The results shown in Figure
3 are from assays performed in industrial (pulp and
paper mills and mining industries) and municipal wastewater
treatment plants. In 15 min, IC50 can be evaluated from
recommended sample concentrations of 100, 50, 25, 12.5,
6.25, and 3.125%.
The tests demonstrate the ability of the LuminoTox device
to provide a rapid screening and monitoring test for toxicity
detection of a broad spectra of molecules found in
industrial or municipal effluents. It offers a fast, on-site
response to the efficacy of a treatment. Standard chemical
testing and bioassays require costly equipment and normally
allow access to data only after several days. For example,
a bioassay on trout takes a minimum of 72 hr of testing.
The LuminoTox technology offers a simple yet effective
method for selecting positive samples. By slightly increasing
the incubation time, it is also possible to increase the
test’s sensitivity to detect very low concentrations of certain
toxic compounds.
Scientific literature, as well as a wide range of validation
tests on different sources of toxic effluents and runoff
water samples, have proven the ability of PECs to react to
a vast array of chemical elements and molecules.1–11
Another critical application of this technology is water
safety. The LuminoTox system is in the process of being
automated in order to provide on-line monitoring of accidental
or intentional toxic discharges into the water supply
or other environmental infrastructures. In light of the ongoing
national focus on security, the technology provides utilieffties with the necessary tools to continue to protect public
health and meet new challenges.
Warning
A few cautionary words are in order. Because the test is
sensitive to pH, light, and temperature, it is very important
to treat the blank, samples, and standards in exactly the
same manner. The test should be performed at a pH
between 6.5 and 7.8 and at temperatures between 4 and 25
°C. Better results are obtained when the blank, samples,
and standards are kept in the same conditions, for example,
at pH 7.8 at 20 °C.
The consumables, or PECs, should be stored in a freezer. PECs
are stable for six months at –20 °C and for two months at 4 °C in
the freeze-dried form, as specified by the manufacturer. Once solubilized,
the PECs are stable for 24 hr at 4 °C or for 5 hr at 20 °C.
The PECs can be frozen once solubilized. It is important to protect
the solubilized PECs from light at all times. If exposed to
light, regeneration is achieved after 15 min in the dark.
References
- Cedeno-Maldonado A, Swader JA, Heath RL. The copper ion as
an inhibitor of photosynthetic electron transport in isolated chloroplasts.
Plant Physiol 1972; 50:698–701.
- de Filippis LF, Hampp R, Ziegler H. The effects of sublethal concentrations
of zinc, cadmium and mercury on Euglana. Arch
Microbiol 1981; 128:407–11.
- Genty B, Briantais JM, Baker NR. The relationship between the
quantum yield of photosynthetic electron transport and quenching of
chlorophyll fluorescence. Biochim Biophys Acta 1989; 990:87–92.
- Conrad R, Büchel C, Wilhelm C, Arselane W, Berkaloff C, Duval
JC. Changes in yield of in-vivo fluorescence of chlorophyll a as a
tool for selective monitoring. J Appl Phycol 1993; 5:505–16.
- Light emission by plants and bacteria. In: Govindjee, Amesz J,
Fork DJ, eds. Orlando, FL: Academic Press Inc., Harcourt Brace
Jovanich, 1986:638.
- Koblizek M, Maly J, Masojidek J. A biosensor for the detection of
triazine and phenylurea herbicides designed using Photosystem II
coupled to a screen-printed electrode. Biotechnol Bioeng 2002;
78(1):110–16.
- Laberge D, Chartrand J, Rouillon R, Carpentier R. In vitro phytotoxicity
screening test using immobilized spinach thylakoid. Env
Toxicol Chem 1999; 18:2851–8.
- Laberge D, Rouillon R, Carpentier R. Comparative study of thylakoid
membranes sensitivity for herbicide detection after physical or
chemical immobilization. Enzyme Microb Technol 2000;
26:332–6.
- Schreiber U, Bilger W. Rapid assessment of stress effects on plant
leaves by chlorophyll fluorescence measurements. In: Tenhunen JD,
ed. Plant response to stress. Berlin, Heidelberg: Springer-Verlag,
1987:27–53.
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis:
the basis. Ann Rev Plant Physiol 1991; 42:313–49.
- Boucher N, Carpentier R. Hg2+, Cu2+ and Pb2+ induced changes in
Photosystem II photochemical yield and energy storage in isolated
thylakoid membranes: a study using simultaneous fluorescence and
photoacoustic measurements. Photosynth Res 1999; 59:164–74.
- Bellemare F, Boucher N, Lorrain L. Method of testing photosynthetic
activities, 2001, WO 2004/046717.
Dr. Boucher, Dr. Lorrain, Ms. Rouette, Ms. Perron, and Dr. Bellemare are
with Lab-Bell Inc., 2263 Ave. du Collège, Shawinigan, Québec,
Canada; tel.: 819-539-8508, ext. 107; fax: 819-539-8880; e-mail: [email protected]. Ms. Déziel is with Centre National en
Electrochimie et Technologies Environnementales (CNETE), and Dr.
Tessier is with the Biology Dept., Collège Shawinigan, Québec, Canada.