Sexual assault kit (SAK) samples account for a significant portion of backlogged cases in forensic laboratories. It can take 4–6 hours to screen SAK evidence, and there may be wide variation among the type and age of samples collected, which can require further analysis time.
If sperm is detected, the sample is processed using differential extraction to separate the suspect sperm fraction from the victim’s epithelial cells in order to improve DNA-based identification downstream. However, many SAKs often do not yield any detectable male DNA when analyzed downstream by autosomal and Y short-tandem repeat (STR) amplification.
As an alternative to time-consuming, labor-intensive differential extraction, the DNA Y-Screen assay (Applied Biosystems, Foster City, Calif. [part of Thermo Fisher Scientific]) assesses swab evidence from SAKs to rapidly detect the presence of a male contributor. In conjunction with other presumptive serological and microscopic slide screening methods, the assay allows the forensic laboratory to confirm serology results, and is an effective tool for detecting male/sperm DNA when slide-screening results are questionable.
Y-Screen assay
The assay, which has been used in previous DNA extraction protocols, including differential extraction,1–3 utilizes NaOH to rapidly lyse cells (including sperm) and the Quantifiler Trio DNA quantification kit (Applied Biosystems) to detect the presence or absence of male DNA prior to standard SAK sample processing. A 7500 Real-Time PCR instrument (Applied Biosystems) with HID Real-Time PCR analysis software v1.2 is used to run the assay. The kit contains primers and probes for several targets—two autosomal human-specific targets (80 and 214 bp), one male human-specific target (75 bp) and an internal positive control target (IPC) (130 bp). The ratio of these quantitative autosomal targets provides a qualitative determination of the level of degradation present in the sample. The IPC in each reaction contains a synthetic DNA template, offers positive confirmation that all assay components are functioning as expected, and is a useful indicator of inhibition present in the sample. All of the human targets in the Quantifiler Trio kit are multicopy targets, which provides for subpicogram-level detection of both human autosomal and male DNA in one assay.
Y-Screen assaying comprises the following steps:
- Cut ~1/10 from each cotton swab sample type.
- Place swab into PrepFiler LySep column assembly (column and sample tube) (Applied Biosystems). Add 100 μL 1N NaOH to the column containing the swab cutting and place in a heat block preheated to 80 °C. Shaking at ~750 rpm is recommended. Incubate at 80 °C for 10 minutes.
- After incubation, centrifuge the column assembly to separate the swab cutting from the lysed cells.
- Add 4 μL glacial acetic acid to the lysed cells in NaOH to neutralize.
- Perform a 1/5 dilution of the neutralized sample by adding 20 μL sample to 80 μL low Tris-EDTA (TE). Alternatively, 400 μL of the low TE can be added directly to the neutralized sample.
- Remove 2 μL of the diluted sample and add to Quantifiler Trio real-time PCR reaction. Use the standard protocol for the Quantifiler Trio kit.
Proof of concept for Y-Screen assay
Mock samples were created by making serial dilutions of either male saliva or seminal fluid and adding 50 μL of sample to female buccal swabs (see Table 1). A small cutting taken from each swab was processed with the Y-Screen assay. The remainder of the swab was processed with a Maxwell 16 nucleic acid extraction instrument (Promega Corp., Fitchburg, Wis.). Purified samples were quantified and genotyped with an Applied Biosystems AmpFlSTR YFiler assay and/or AmpFlSTR Identifiler Plus assay.
Table 1 – Y-Screen assay results and corresponding STR results with YFiler and/or Identifiler Plus kits
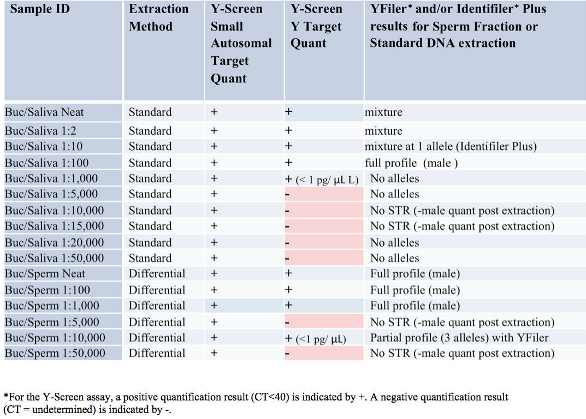
As shown in Table 1, with 50 μL neat saliva from a male donor added to a buccal swab from a female donor, the assay was positive for both the small autosomal target and the Y target. Post-extraction, the purified DNA was quantified at 14.5 ng/μL with a male:female ratio of 1:1.4. The resulting Identifiler Plus STR profile (Figure 1) shows the expected mixture profile. As the saliva input dilution decreased, the amount of male DNA decreased, as expected. With the 1:1000 dilution, the Y-Screen assay was still positive with subpicogram levels of male DNA.
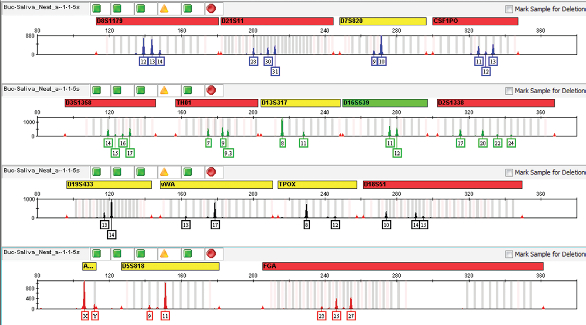 Figure 1 – Identifiler Plus results for purified DNA from a buccal sample from a female donor containing 50 μL saliva from a male donor.
Figure 1 – Identifiler Plus results for purified DNA from a buccal sample from a female donor containing 50 μL saliva from a male donor.After DNA extraction, 1.6 pg/ μL male DNA was recovered and no alleles were detected with the YFiler assay (Figure 2). For the dilutions to 1:50,000, no male DNA was obtained with the Y-Screen assay and no information was obtained with STR following purification. In all mock samples tested, the Y-Screen assay was predictive of downstream results; a positive male quantification result with Y-Screen correlated with a positive male quantification result post-extraction.
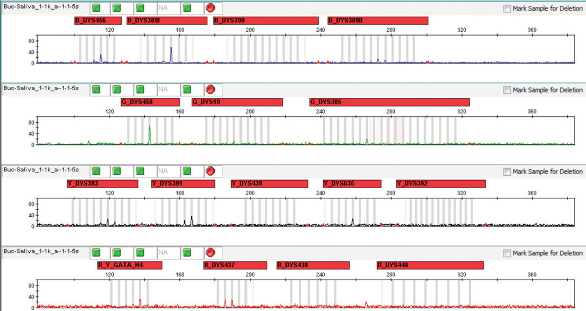 Figure 2 – YFiler results for purifi ed DNA from a buccal sample from a female donor containing an equivalent of 0.05 μL of saliva sample from a male donor.
Figure 2 – YFiler results for purifi ed DNA from a buccal sample from a female donor containing an equivalent of 0.05 μL of saliva sample from a male donor.Y-Screen assay with mock casework samples
A set of 20 mock casework samples was created with a range of bodily fluids including sperm (seminal fluid, SF) diluted in TE-4, blood, saliva and touch (see Table 2). Mixtures 1–4 were buccal swabs from a female donor with sperm diluted as described and 50 μL of each dilution of sperm added. Mixtures 6 and 8–10 were prepared with 40 μL whole female blood and an equal amount of the listed seminal fluid dilution. Mixture 7 was similarly prepared using 60 μL of female blood and 20 μL of a diluted seminal fluid. Mixture 5 was a previously prepared training sample for which the volumes were not recorded. Samples Touch 2 and Touch 4 were neat male saliva on a swab rubbed on a target arm and then collected with a fresh swab. Samples Touch 1 and 3 were swabbings collected from an individual’s arm after being grabbed on the arm by another participant.
Table 2 –Y-Screen assay results and corresponding STR results with YFiler and/or Identifiler Plus kits
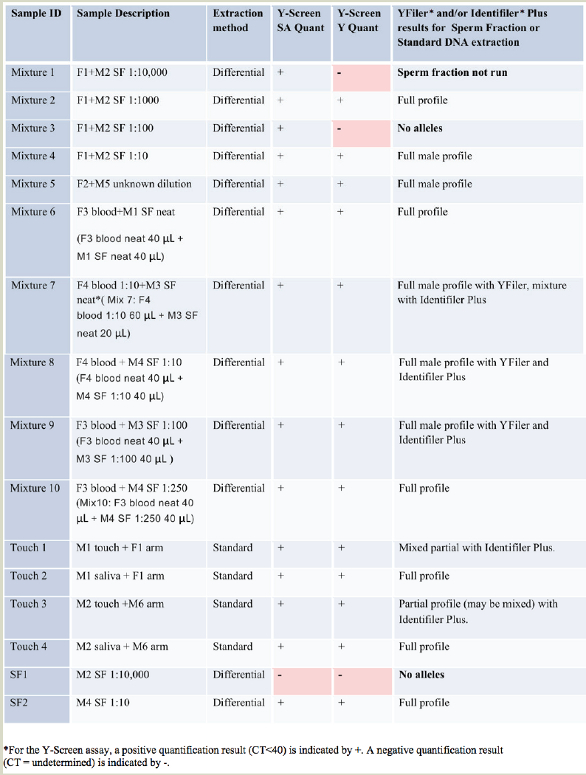
As with the previous dilution series data, in all cases the Y-Screen assay was predictive of downstream results; a positive male quantification result with Y-Screen correlated with a positive male quantification result postextraction. For the three samples with a zero male quantification result, no samples provided useful STR information. For Mixture 1, the sample was not amplified because current laboratory practice for casework is to stop at quantification if the result is zero.
Discussion
The protocol described supports only swab samples and was not tested on other substrates by the Utah Bureau of Forensic Services (UBFS). All swabs are known to have variable amounts of cellular material and free DNA across the swab itself, which is also a problem for serological and slide screening methods. For this reason, the assay must be validated with a uniform cutting procedure to help reduce the effects of sample heterogeneity. Some users have taken multiple cuttings of the same swab to improve yields and accuracy.
While very low quantification results with the Y-Screen assay generally correlate to low quantification results postextraction, this is not always the case for all samples. Therefore, it is not recommended to make direct correlations between the pre-extraction (Y-Screen) and postextraction quantification values. Any inhibitors present in the Y-Screen lysate from samples may impact the IPC and degradation index results, but are typically removed through standard differential extraction cleanup methods. Quantifiler Trio using HID Real-Time PCR analysis software v1.2 provides clear warning signs of sample and reaction quality when the Y-Screen results are difficult to interpret, information that is not available with traditional serological assays.
Conclusion
Due to high backlogs of SAK samples, laboratories must make critical decisions about which samples to process. Sample screening using traditional serological assays and microscopic sperm slide searches are done early in the workflow. The DNA-based Y-Screen assay, using Quantifiler Trio and HID Real-Time PCR analysis software v1.2, enhances these screening methods with intelligent sample quantity and quality interrogation tools. Moreover, correlation between pre-extraction quantification results and downstream STR results has been demonstrated.
References
- Hudlow, W.R. and Buoncristiani, M.R. Development of a rapid, 96-well alkaline based differential DNA extraction method for sexual assault evidence. Forensic Sci. Int. Genet. 2012, 6(1), 1–16.
- Rudbeck, L. and Dissing, J. Rapid, simple alkaline extraction of human genomic DNA from whole blood, buccal epithelial cells, semen and forensic stains for PCR. Biotechniques 1998, 25(4), 588–92.
- Truett, G.E.; Heeger, P. et al. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 2000, 29(1), 52–4.
Allison Holt is staff scientist, and Sheri Olson is senior product manager, Thermo Fisher Scientific, 180 Oyster Point Blvd. East, South San Francisco, Calif. 94090, U.S.A.; tel.: 650-876-1949; e-mail: [email protected]; www.thermofisher.com. Michele Marfori is forensic scientist quality manager, and Denise Yong Ning Oh is forensic scientist 1, Utah Department of Public Safety, Salt Lake City, Utah, U.S.A. The data presented were generated by the Utah Bureau of Forensic Services [UBFS]. Quantifiler Trio, 7500 Real-Time PCR instrument, HID Real-Time PCR software v1.2, PrepFiler LySep, AmpF1STR YFiler and AmpF1STR Identifiler Plus are for forensic or paternity use only, unless stated otherwise on individual product labeling.