Prof. Seiji Takeda leads a research group in the Department of Nanocharacterization for Nanostructures and Functions of the Nanoscience and Nanotechnology Center at the Institute of Scientific and Industrial Research, Osaka University. Prof. Takeda’s research includes the development and application of environmental transmission electron microscopy (ETEM) techniques for the characterization of nanostructures.
Osaka University is a major research institution with 11 undergraduate schools, 16 graduate schools, and five research institutes. The Institute of Scientific and Industrial Research (ISIR) conducts world-class interdisciplinary research in the fields of materials, information, and biological sciences. It plays a leading role in nanoscience and nanotechnology research through its Nanoscience and Nanotechnology Center, which was established in 2002 and was Japan’s first such center attached to a university.
In addition to his work in ETEM instrumentation and techniques and the work described here in catalytic reactions on the metal nanoparticle catalysts, Prof. Takeda’s research interests include atomic scale in situ observation of the growth of carbon nanotubes, the fabrication of novel nanostructures in semiconducting materials by means of self-organized growth and electron irradiation, the mechanism of the nucleation and growth of lattice defects during the growth of semiconducting crystal films, and the characterization of lattice defects introduced by ion-implantation and electron irradiation.
In a publication1 summarizing his group’s work with the Titan ETEM (FEI, Hillsboro, OR), Prof. Takeda identified five issues that need to be addressed to establish a firm experimental foundation for quantitative in situ ETEM observations of materials operating in gases. These issues include:
- Developing a robust, easy-to-use ETEM for high spatial resolution in well-controlled environments
- Quantifying the effects of intense electron irradiation
- Understanding the significance of microstructural heterogeneity observed in real catalyst particles
- Bridging the temperature and pressure gaps between atmospheric and high vacuum conditions
- Imaging atoms and molecules during catalytic reactions.
Prof. Takeda and his colleagues addressed these issues in a series of experiments observing the catalytic oxidation of carbon monoxide by gold nanoparticles supported on titanium oxide and cerium oxide supports.
ETEM system
 Figure 1 – Titan ETEM G2 installed at the facility.
Figure 1 – Titan ETEM G2 installed at the facility.Characterizing the performance of the Titan ETEM G2 system installed in his lab (Figure 1), Prof. Takeda stated that even at 200 kV the instrument has demonstrated an information limit of 0.12 nm at gas pressures above 1000 Pa. He also pointed out that the software-controlled operation is fast and simple and confers a very real benefit by enabling the easy acquisition of substantial, systematic, and reproducible high-resolution ETEM data. Because of this, he hopes more people will be interested in in situ research.
Electron irradiation effects
Catalyst systems, consisting of the catalyst particles and gas molecules, under observation in an ETEM are heavily bombarded by high-energy electrons. The energies of the electrons dwarf the energy transferred between catalysts and gas molecules in an ordinary catalytic reaction, making it impossible to ignore the potential effects of irradiation in quantitative ETEM observations of in situ catalyst chemistry. Prof. Takeda quantified these effects by measuring their dependence on electron current density and total electron dose and extrapolating back to zero.2 In this way, he was able to diagram the evolution of irradiation-induced structural changes and establish limits on dose and density below which the particles retained their intrinsic morphologies (Figure 2). Based on estimates of the rates of oxidation, adsorption, and desorption of CO molecules on the particle surfaces, he was also able to confirm that catalytic reactions were occurring during the ETEM observations.
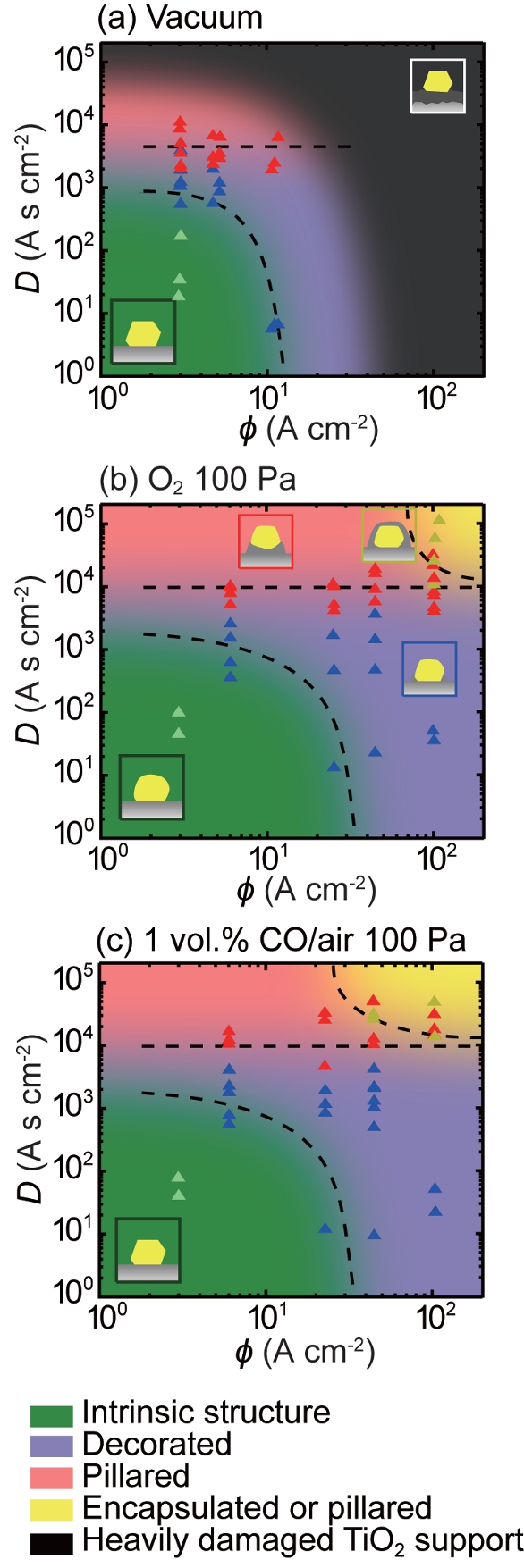 Figure 2 – Structure evolution diagrams of Au/ TiO2 catalysts under electron irradiation as a function of electron current density, f, and dose, D, in a) vacuum, b) O2 (100 Pa), and c) 1 vol% CO in air (100 Pa) at room temperature. The intrinsic structures were observed in the green regions, while gold nanoparticles (GNPs) with decorations and pillars were observed in the blue and red regions, respectively. In the yellow regions, either GNPs with pillars or encapsulated GNPs were observed. A blue, red, or yellow triangle indicates the D at which decoration, pillar, or encapsulation around a GNP was first detected at a given current density. In vacuum, TiO2 supports were heavily damaged, as shown in the black region in (a).2
Figure 2 – Structure evolution diagrams of Au/ TiO2 catalysts under electron irradiation as a function of electron current density, f, and dose, D, in a) vacuum, b) O2 (100 Pa), and c) 1 vol% CO in air (100 Pa) at room temperature. The intrinsic structures were observed in the green regions, while gold nanoparticles (GNPs) with decorations and pillars were observed in the blue and red regions, respectively. In the yellow regions, either GNPs with pillars or encapsulated GNPs were observed. A blue, red, or yellow triangle indicates the D at which decoration, pillar, or encapsulation around a GNP was first detected at a given current density. In vacuum, TiO2 supports were heavily damaged, as shown in the black region in (a).2Heterogeneity of real catalysts
Gold nanoparticles in real catalysts prepared by chemical synthesis can have very narrow size distributions, but exhibit significant microstructural heterogeneity (Figure 3).2 The issue is whether a catalyst particle being observed at high resolution is responsible for catalytic activity or not. Since there is no microscopic method to directly determine the catalytic activity of a small region of a real particle during ETEM observation, the solution requires a statistical analysis of large amounts of ETEM data characterizing the behavior of the particle population.
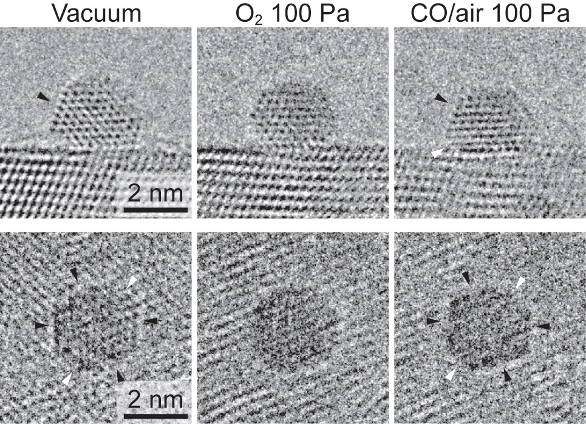 Figure 3 – Systematic morphology change of gold nanoparticles in Au/TiO2, depending on the environment. The images were recorded using a Cs-corrected Titan ETEM G2.2
Figure 3 – Systematic morphology change of gold nanoparticles in Au/TiO2, depending on the environment. The images were recorded using a Cs-corrected Titan ETEM G2.2Prof. Takeda was able to show that the statistical distribution of gold nanoparticles into different structural categories was a function of the pressure and composition of the gas environment.3 In a mixture of CO and O2, more particles assume a faceted morphology at higher total gas pressures (P(CO) + P(O2)) and higher concentrations of CO (P(CO)/P(O2)) (Figure 4). As these values decrease, the particles demonstrate a reversible transition to a distribution dominated by rounded particles. This was the first time these systematic and quantitative microstructural data have been obtained for an operating nanoparticle catalyst. The stability of the Titan ETEM G2 played an important enabling role in collecting the large data sets.
 Figure 4 – Morphology phase diagram of gold nanoparticles supported on CeO2, as a function of the total pressure of the gases (CO and O2), P(CO) + P(O2), and the ratio of partial pressures P(CO)/P(O2). The circles, triangles, and squares represent rounded, statistically mixed, and faceted morphologies, respectively.3
Figure 4 – Morphology phase diagram of gold nanoparticles supported on CeO2, as a function of the total pressure of the gases (CO and O2), P(CO) + P(O2), and the ratio of partial pressures P(CO)/P(O2). The circles, triangles, and squares represent rounded, statistically mixed, and faceted morphologies, respectively.3Prof. Takeda determined that CO molecules adsorb preferentially to, and thereby stabilize, the major {111} and {100} facets.3 Studies suggest that oxygen atoms (catalytically dissociated from O2 gas and possibly promoted by electron irradiation) can be adsorbed on both flat and defective surfaces of gold nanoparticles and on both major and minor {110} facets. The rounded morphology observed in O2 gas is actually a fluctuating surface caused by the destabilizing influence of active oxygen species in the absence of stabilizing CO molecules. He concluded that the reversible morphology change of gold nanoparticles supported on CeO2 and TiO2 correlated well with their catalytic activity and may serve as a valuable indicator of catalytic potential in the search for new catalysis systems.
Pressure and temperature gaps
A pressure gap certainly exists between observations made at atmospheric pressure and those made in a conventional high-vacuum TEM. ETEM experiments extend experimental pressures up to 2500 Pa (about 2.5% of atmospheric pressure). With Cs correctors, the Titan ETEM now extends atomic-scale observations above 2000 Pa (Figure 5).4 In practice, gold nanoparticles for carbon monoxide oxidation are used at partial pressures (P(CO)) ranging from 1 to 10 Pa, a range fully covered by ETEM. As for the temperature gap, gold nanoparticle catalysts are highly active even below room temperature. ETEM is also capable of observations at elevated temperatures, as high as 1000 °C with special holders. Therefore, neither a pressure gap nor a temperature gap exists.
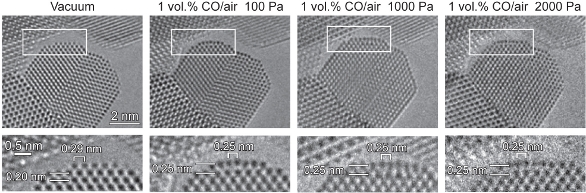 Figure 5 – No pressure gap. The Au{100}-hex reconstructed surface remained stable under CO gas with lower (100 Pa) to higher (2000 Pa) pressures of 1 vol% CO/air (Au/CeO2). The images of the rectangular regions are enlarged and shown at the bottom. The images were recorded using a Cs-corrected ETEM.4 (Reproduced with permission from Ref. 4.)
Figure 5 – No pressure gap. The Au{100}-hex reconstructed surface remained stable under CO gas with lower (100 Pa) to higher (2000 Pa) pressures of 1 vol% CO/air (Au/CeO2). The images of the rectangular regions are enlarged and shown at the bottom. The images were recorded using a Cs-corrected ETEM.4 (Reproduced with permission from Ref. 4.)Imaging atoms and molecules during catalytic reactions
Having concluded that the reversible morphology change exhibited by gold nanoparticles was associated with their catalytic ability, Prof. Takeda wanted to observe individual atoms and molecules participating in catalytic reactions. Choosing particles that exhibited the change, he acquired high-resolution images under vacuum and reaction conditions and found distortions in the two topmost layers of the {100} facets in a reaction environment (Figure 6).4 Having previously determined that CO molecules dominate the surface of gold nanoparticles under reaction conditions, he concluded that the changes in these topmost layers were likely induced by the interaction of CO molecules and gold atoms. ETEM visualization of CO molecules adsorbed to the surface (Figure 7) and confirmation of the surface structure by image simulations and ab initio calculations revealed that the changes in surface structure of the {100} facets allowed them to adsorb CO molecules at a significantly higher density.
 Figure 6 – Au{100}-hex reconstructed surface under catalytic conditions. A gold nanoparticle supported on CeO2 in (a) vacuum and (b) a reaction environment (1 vol% CO in air at 45 Pa) at room temperature. The two {100} facets in the rectangular regions indicated by I and II are shown enlarged in vacuum and in CO in air. The images were recorded using a Cs-corrected ETEM.4 (Reproduced with permission from Ref. 4.)
Figure 6 – Au{100}-hex reconstructed surface under catalytic conditions. A gold nanoparticle supported on CeO2 in (a) vacuum and (b) a reaction environment (1 vol% CO in air at 45 Pa) at room temperature. The two {100} facets in the rectangular regions indicated by I and II are shown enlarged in vacuum and in CO in air. The images were recorded using a Cs-corrected ETEM.4 (Reproduced with permission from Ref. 4.)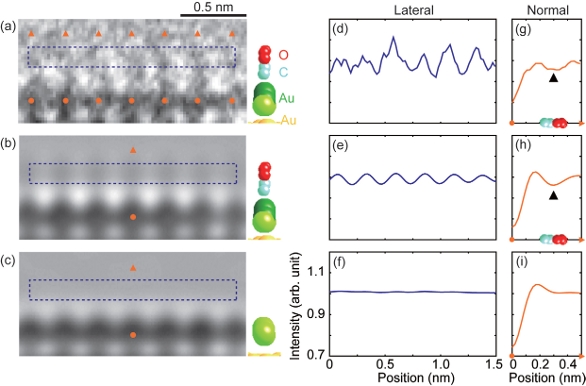 Figure 7 – A glimpse of gas molecules on the surface of catalysts under reaction conditions: a) Observed and b) simulated image of the Au {100}-hex reconstructed structure with CO molecules. c) Simulated image of Au{100}-hex reconstructed structure without CO molecules. Lateral intensity scans of (a), (b), and (c) are shown in (d), (e), and (f), respectively. Line scans normal to the surface of (a), (b), and (c) are shown in (g), (h), and (i), respectively. The oscillation intensity in observation (d) can be accounted for by considering the adsorbed CO molecules, as shown in (e). The Au{100}-hex reconstructed surface without CO molecules could not reproduce the oscillating intensity, as shown in (f). The images were recorded using a Cs-corrected ETEM.4 (Reproduced with permission from Ref. 4.)
Figure 7 – A glimpse of gas molecules on the surface of catalysts under reaction conditions: a) Observed and b) simulated image of the Au {100}-hex reconstructed structure with CO molecules. c) Simulated image of Au{100}-hex reconstructed structure without CO molecules. Lateral intensity scans of (a), (b), and (c) are shown in (d), (e), and (f), respectively. Line scans normal to the surface of (a), (b), and (c) are shown in (g), (h), and (i), respectively. The oscillation intensity in observation (d) can be accounted for by considering the adsorbed CO molecules, as shown in (e). The Au{100}-hex reconstructed surface without CO molecules could not reproduce the oscillating intensity, as shown in (f). The images were recorded using a Cs-corrected ETEM.4 (Reproduced with permission from Ref. 4.)Conclusion
Prof. Takeda’s work has demonstrated the ability of current-generation ETEM to perform quantitative in situ microscopy with atomic scale spatial resolution. In particular, he has systematically identified and addressed a number of crucial issues to lay a solid foundation for the advancement of the technique. The highresolution ETEM techniques he has pioneered will help researchers identify catalytically active sites in reaction environments and will certainly play an important role in future contributions to catalytic chemistry.*
*Prof. Takeda would like to acknowledge his colleagues at Osaka University, especially Dr. H. Yoshida, Dr. Y. Kuwauchi, and Mr. T. Uchiyama, for their extremely elaborate ETEM experiments and analyses. He would also like to acknowledge Dr. M. Haruta for his collaboration in the studies.
References
- Takeda, S.; Yoshida, H. Atomic-resolution environmental TEM for quantitative in-situ microscopy in materials science. Microscopy 2013, 62,193–203. doi 10.1093/jmicro/dfs096.
- Kuwauchi, Y.; Yoshida, H. et al. Intrinsic catalytic structure of gold nanoparticles supported on TiO2. Angew. Chem. Int. Ed. 2012, 51, 7729–33.
- Uchiyama, T.; Yoshida, H. et al. Systematic morphology changes of gold nanoparticles supported on CeO2 during CO oxidation. Angew. Chem. Int. Ed. 2011, 50, 10157–60.
- Yoshida, H.; Kuwauchi, Y. et al. Visualizing gas molecules interacting with supported nanoparticulate catalysts at reaction conditions. Science 2012, 335, 317–19.
Joerg Jinschek is Product Marketing Manager, FEI, 5350 NE Dawson Creek Dr., Hillsboro, OR 97124, U.S.A.; tel.: 503-726-7500; e-mail: [email protected]; www.fei.com.