Electron paramagnetic resonance spectroscopy (ESR) is a spectroscopic technique that permits the detection of unpaired electrons originating from radicals, including those induced by irradiation or from paramagnetic ions in an applied external magnetic field. A typical ESR spectrum from an irradiated biological substance was first reported in 1955.1 In 1971, ESR was considered a detection tool for irradiated food.2
 Figure 1 – ESR spectra of radiation-induced radicals: a) cellulose radical, b) crystalline sugar radical, c) hydroxyapatite radical.
Figure 1 – ESR spectra of radiation-induced radicals: a) cellulose radical, b) crystalline sugar radical, c) hydroxyapatite radical.ESR has been accepted as a standard method by the European Committee for Standardization (CEN). European Standards EN 1787:2000 (cellulose),3 EN13708:2001 (crystalline sugar),4 and EN 1786:2001 (bone)5 are based on electron spin resonance. The European Standards have been adopted by the Codex Alimentarius Commission.6
An ESR spectrum of the radiation-induced free radicals can be one of three types (Figure 1):
- Cellulose (cellulosic) radicals in food materials of plant origin containing cellulose such as seeds, peels, some herbs, and spices
- Sugar radicals from dried fruits containing D-fructose, D-glucose, and D-saccharose as the main components
- Hydroxyapatite radicals produced from bone-containing foods; CO2–1, CO3–3, and CO3–1 are the key carbonate-derived radicals that define typical radiation-induced spectra.
Principle of ESR
In principle, ESR finds paramagnetic centers (e.g., radicals) that may or may not be radiation induced. A strong external magnetic field generates a difference between the energy levels of the electron spins, ms = +½ and ms = –½, which results in resonance absorption of an applied microwave energy (Figure 2). Measurement of the g value of the ESR signal, which is the frequency to the magnetic field strength values ratio (hν/βB0, where h is Planck’s constant, ν is the microwave frequency, β is the Bohr magneton, and B0 is the magnetic field), is used for the identification of irradiated samples. The ESR spectra are presented as the first derivative of the absorption with respect to the applied magnetic field (EN 1787, 2000).3 Radiation-induced paramagnetic species can remain stable in the rigid and dehydrated parts of a food sample for a long time in comparison to the shelf life of that food.
 Figure 2 – Principle of electron spin resonance spectroscopy for the detection of irradiated food and energy level of electrons in a magnetic field.
Figure 2 – Principle of electron spin resonance spectroscopy for the detection of irradiated food and energy level of electrons in a magnetic field.Applications of ESR
In food materials of plant origin, irradiation can generate free radicals in cellulose and crystalline sugars, which could serve as irradiation detection markers in ESR analysis.7 Radiation-induced radicals generally occur in the solid and dry fractions of food. The rigid structure of the matrix is able to trap free radicals or excited states of the electrons and inhibit them to react with each other or with food components present in wet portions of the food. Foods containing bone, seeds, shells, etc., have low moisture content, and radicals remain sufficiently stable for ESR analysis. In general, it is difficult to apply the ESR technique to foods containing high-moisture content, since free radicals produced during the irradiation process disappear very rapidly.
ESR spectroscopy can also be utilized for the identification of irradiated fruits and vegetables. 8 In high-moisture products like fruits and vegetables, the irradiation-induced radicals are not stable; however, hard parts that hold low moisture can be utilized to detect irradiation treatment because the free radicals are relatively stable there. Raffi and others studied the ESR signal of strawberry seeds; the signal increased with irradiation dose and was largely affected by water content.9
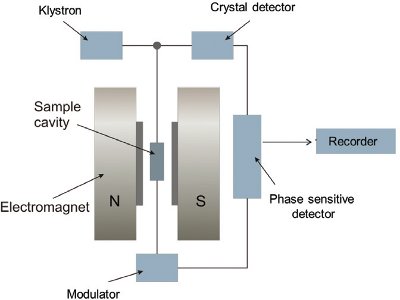 Figure 3 – Block diagram of a simple ESR spectrometer.
Figure 3 – Block diagram of a simple ESR spectrometer.For ESR measurements, the sample is placed in a quartz ESR tube (5 mm diam); the tube is sealed with paraffin film and stored in the dark in a desiccator at 40 ± 5% relative humidity. The sample is subjected to the simultaneous action of a magnetic field and an electromagnetic microwave of very high frequency (Figure 3). ESR signals are measured as described in the European Standard Protocols.3–5
A number of research studies have reported the radiation-induced signals in plant materials containing cellulose with two side peaks having g values (g =2.0201 and g =1.9851), linked with a central signal (Figure 1a).10 On the other hand, irradiated sugar standard markers showed the multicomponent unparalleled radiation-induced signals that are characteristic of the hydroxyalkyl radical in crystalline carbohydrates as reported in irradiated rice noodles,11 irradiated wheat,12 dried mushrooms,13 and dried fruits (Figure 1b).4
Barabas et al. reported the nature of paramagnetic centers in marine carbonates.14 CO2–1, CO3–3, and CO3–1 are key carbonate-derived radicals used to define typical radiation-induced spectra. In particular, generation of CO2–1 from carbonated materials upon irradiation is important with g = 1.9996, which is more specific than other radicals (Figure 1c).
Effect of drying treatment on ESR spectra
Effective drying techniques are required as pretreatments to reduce the moisture content in order to obtain clear or improved ESR spectral features 15 Recently, Akram et al. reported the significant effects of different sample pretreatments such as freeze drying, alcohol extraction, and water-alcohol extraction to characterize different irradiated sauces using the ESR technique (Figure 4).16 The alcohol extraction pretreatment of irradiated sauce samples demonstrated improved applicability of the ESR technique in comparison to the freeze drying pretreatment.
 Figure 4 – Effects of different sample pretreatments on ESR signals from 10 kGy irradiated tomato ketchup. FD, freeze drying; AE, alcohol extraction; WAE, water washing followed by alcohol extraction. (Reproduced with permission from Ref. 16.)
Figure 4 – Effects of different sample pretreatments on ESR signals from 10 kGy irradiated tomato ketchup. FD, freeze drying; AE, alcohol extraction; WAE, water washing followed by alcohol extraction. (Reproduced with permission from Ref. 16.)Previously, de Jesus et al. reported water extraction by alcohol for fruit pulps of kiwi, papaya, and tomato containing high moisture content.7 In another study, de Jesus et al. observed the dose-dependent increase in radiation-induced cellulose radical signals in the flesh of irradiated vegetables analyzed by the ESR technique after different sample pretreatments.17 Similarly, nitric acid hydrolysis was found to be the most suitable treatment for better ESR detection in irradiated sprout seeds.18 Freeze drying effectively reduced the moisture content but did not result in any compositional changes in the matrix. On the other hand, ethyl alcohol extraction was also unable to separate the residual solid from complex materials. However, acid hydrolysis proved successful in digesting the interfering compounds with a weak acid concentration (5%) in sprout seed samples, and cellulose or crystalline sugar-based ESR signals were detected upon analysis (Figure 5).
 Figure 5 – ESR spectra of irradiated broccoli sprout seeds after nitric acid (5%) extraction.
Figure 5 – ESR spectra of irradiated broccoli sprout seeds after nitric acid (5%) extraction.Lee et al. compared the ESR spectral characteristics of radiation-induced radicals in irradiated standard materials (cellulose, sugars, and hydroxyapatite) using different ESR spectrometers, such as the JES-300 (JEOL, Tokyo, Japan), Miniscope MS-400 (Magnettech, Berlin, Germany), and e-scan™ food analyzer (Bruker, Billerica, MA).19 All irradiated standard marker materials gave characteristic radiation-induced ESR signals that were distinguishable based on their shape and respective g values. However, each standard marker material showed different sensitivity to ESR detection corresponding to the composition and crystallinity of saccharides. Instrumental variations in signal intensity, spectral shapes, and detection applicability were observed and should be taken into consideration from a practical point of view.
Advantages and limitations of ESR
ESR has become increasingly popular all over the world, and efforts are being made by researchers to extend the application of ESR methodology to identify several types of irradiated food. ESR is a nondestructive technique that can detect paramagnetic ion and free radicals in a variety of materials. ESR is specific, quick, and user friendly, and can also be employed for quantitative estimation. The technique has also been used successfully for herbs, nuts, spices, and meat. However, ESR results are affected by the nature of food and its water content.
References
- Gordy, W.; Ard, W.B. et al. Microwave spectroscopy of biological substances. I. Paramagnetic resonance in X-irradiated amino acids and proteins. Proc. Natl. Acad. Sci. USA 1955, 41, 983–4.
- Boshard, J.A.P.; Holmes, D.E. et al. An inherent dosimeter for irradiated foods: papayas. Appl. Radiat. Isotopes 1971, 22, 316–18.
- Foodstuffs—Detection of Irradiated Food Containing Cellulose by ESR Spectroscopy; EN1787; European Committee of Standardization (CEN): Brussels, Belgium, 2000.
- Detection of Irradiated Food Containing Cellulose by ESR Spectroscopy; EN13708; European Committee of Standardization (CEN): Brussels, Belgium, 2002.
- Detection of Irradiated Food Containing Cellulose by ESR Spectroscopy; EN1786; European Committee of Standardization (CEN): Brussels, Belgium, 1997.
- General Methods for the Detection of Irradiated Foods; CODEX STAN 231-2001, Rev. 1-2003; CAC 2003; Codex Alimentarius Commission: Rome, Italy.
- Delincée, H.; Soika, C. Improvement of the ESR detection of irradiated food containing cellulose employing a simple extraction method. Radiat. Phys. Chem. 2002, 63, 437–41.
- de Jesus, E.F.O.; Rossi, A.M. et al. An ESR study on identification of gamma-irradiated kiwi, papaya and tomato using fruit pulp. Int. J. Food Sci. Technol. 1999, 34, 173–8.
- Raffi, J.J.; Agnel, J.P.L. et al. Electron spin resonance identification of irradiated strawberries. J. Chem. Soc. Faraday T. 1988, 84, 3359–62.
- Raffi, J.; Stocker, P. Electron paramagnetic resonance detection of irradiated foodstuffs. Appl. Magn. Reson. 1996, 10, 357–73.
- Sudprasert, W.; Sahakan, M. et al. Identification of irradiated rice noodles by electron spin resonance spectroscopy. Radiat. Meas. 2012, 47, 640–3.
- Shimoyama, Y.; Ukai, M. et al. ESR detection of wheat flour before and after irradiation. Spectrochim. Acta A 2006, 63, 888–90.
- Malec-Czechowska, K.; Strzelczak, G. et al. Detection of irradiation treatment in dried mushrooms by photostimulated luminescence, EPR spectroscopy and thermoluminescence measurements. Eur. Food Res. Technol. 2003, 216, 57–65.
- Barabas, M.; Mudelsee, M. et al. Dose-response and thermal behavior of the ESR signal AT g = 2.0006 in carbonates. Quat. Sci. Rev. 1992, 11, 173–9.
- Akram, K.; Ahn, J.J. et al. Analytical Methods for the Identification of Irradiated Foods. In: Ionizing Radiation: Applications, Sources and Biological Effects; Belotserkovsky, E.; Ostaltsov, Z., Eds.; Nova Science Publishers: New York, NY, 2012; pp 1–36.
- Akram, K.; Ahn, J.J. et al. Characterization of electron spin resonance spectroscopy of irradiated sauces with different sample treatment. Food Chem. 2013, 138, 1878–83.
- de Jesus, E.F.O.; Rossi, A.M. et al. Identification and dose determination using ESR measurements in the flesh of irradiated vegetable products. Appl. Radiat. Isotopes 2000, 52, 1375–83.
- Shahbaz, H.M.; Kwon, J.H. et al. In: Improved Electron Spin Resonance Spectroscopy with Different Sample Treatments to Identify Irradiated Sprout Seeds. Proceedings Book of 17th International Meeting of Radiation Processing: Shanghai, China; p 187.
- Lee, J.H.; Ahn, J.J. et al. Comparison of ESR spectra of irradiated standard materials using different ESR spectrometers. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 407−11.
The authors are with the School of Food Science & Biotechnology, Kyungpook National University, Daegu 702-701, Republic of Korea; tel.: +82 53 950 5775; fax: +82 53 950 6772; e-mail: jhkwon@ knu.ac.kr.