Boron-doped diamond (BDD), a
working electrode material (ESA
Biosciences,
Chelmsford, MA)
developed for use with HPLC–electrochemical detection, promises to
open new areas for analysis with electrochemical detectors. Electrochemical
detection (ECD) with HPLC has
proven to be of significant value in measuring
biologically and clinically relevant
molecules. ECD detectors work
by applying a voltage between a working
and a reference electrode in a flow
cell. As molecules pass through the flow
cell, those that can be easily oxidized or
reduced at the applied potential react at
the working electrode, producing a flow
of electrons (Figure 1). The detector
(Figure 2) measures this flow of electrons.
With the electronics available
today, it is a highly sensitive measurement.
Only those molecules that will
oxidize (or reduce) at the electrode at
the applied potential are detected, thus
giving high selectivity. Because of the
inherent high sensitivity, selectivity,
and wide dynamic range of the technique,
it is used extensively in important
areas such as brain research and for
the diagnosis of specific cancers.

Figure 1 - In an electrochemical detector, a potential is applied between a working and reference electrode. The molecule of interest is oxidized or reduced at the working electrode. The result is a flow of electrons (current) that can be measured and quantified. This requires that the molecule be oxidized or reduced at the set potential.

Figure 2 - Coulochem III electrochemical detector (ESA Biosciences). The electronics control and hold the potential applied to the electrodes in the flow cell. The Coulochem III collects and processes the signal and sends it to the data station of an HPLC system.
Over the years, a number of materials
have been used for the working electrodes.
These include noble metals (such
as Au, Ag, and Pt) and various forms
of carbon (carbon paste, graphite, and
amorphous or glassy carbon). The carbon-based electrodes are considered general-purpose working electrodes and have
found extensive use in a broad array of
applications.1 All of these carbon-based
electrodes share a common microstructure
and demonstrate similar behavior.
Despite the high utility of such graphitic
and glassy carbon electrodes,
they are limited in the molecules they
can detect because of the restrictions
in the potential ranges that can be used
with such materials. (They may even
require high overpotential to produce a
response—some compounds simply do
not react well; other reactions actually
require the working electrode to take
part in the reaction mechanism, rather
than just acting as an electron source
or sink.) Additionally, some chromatographic
conditions and sample matrices
cause degradation in the electrode’s
performance that is not easily restored
during or following the analysis. This
is often the case resulting from
adsorption of contaminants in
the sample or even the analyte
onto the electrode surface. An
example of such an application
is the analysis of thiols and disulfides
in biological samples.
An ideal working electrode
that can extend the utility and
robustness of ECD would have
many of the properties of the
carbon electrodes but would be
able to operate at more extreme
(either higher or lower) potentials
than typical carbon-based
electrodes without suffering
from the high noise resulting
from oxidation of water in the
mobile phase.
During the 1980s, various forms of carbon
became widely used as electrodes
for general electrochemistry. This was
due to the simplicity of fabrication, the
relatively low cost, and the ability to
produce electrodes of high surface area.
The applications included electroanalysis,
energy storage devices, and electrosynthesis
and reaction, as well as flow
injection analysis and HPLC with ECD.
In the mid-1980s, techniques were
developed for low-pressure diamond
synthesis. Although diamond is a
mechanically resilient and strong material,
unfortunately, with its SP3 orbital
structure, it is notoriously inert and
unsuitable for use as a working electrode
material. Fortunately, methods became
available for the inclusion of metal dopants
in these diamond films, rendering
the inherently insulating diamond film
conductive. One such dopant is boron.
Typically, electrodes of boron-doped
diamond are constructed on a supporting substrate, often silica, glassy carbon,
or metals. The polycrystalline, thin film
is formed by chemical vapor deposition.
Considerable work has been published,
originally on the properties of
these materials and later on their use
in numerous applications. Pioneering
work in the early 1990s was conducted
by Swain2,3 and colleagues in
the U.S.A., and Fujishima4,5 and co-workers
in Japan. Despite the extensive
and impressive work of these
groups as well as others, the use of
BDD material for analytical electrochemistry
has remained primarily in
research laboratories.
The BDD electrode has several features
that make it a favorable working electrode
material for use in HPLC–ECD.
As previously noted, diamond itself is an
excellent insulator. When moderately
doped with boron, the material behaves
as a semiconductor, but at high levels of
boron doping, diamond takes on metal-like
properties, making it a suitable material
for a working electrode. BDD electrodes
have low capacitance, resulting in
lower inherent noise, a uniform surface,
high chemical and structural stability,
and resistance to fouling. When used
as an electrode, the BDD electrode can
operate with a wider range of working
potentials than glassy carbon.
Enhanced surface stability
The surface stability of the diamond
makes it resistant to surface modification.
It is common for thin-film carbon-based electrodes to change their
properties over time (e.g., oxygen termination versus hydrogen termination,
etc.), requiring extensive
polishing or electrochemical
processing to restore the original
behavior. Even at high potentials
the surface of the BDD working
electrode remains inert and has a
long working life without changing
its characteristics. Because of
the inherent characteristics of the
surface, there is little or no fouling
due to adsorption of contaminants
from the analyte or sample matrix.
“Chip” electrode design
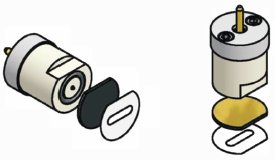
Figure 3 - The BDD electrode is configured on a replaceable chip. The chip and gasket are placed in the cell body and are held in place with the pin assembly to make electrical contact and create a liquid seal in the flow cell.
To take advantage of the properties
of the BDD electrode, a thin-film
amperometric cell design was chosen.
The cell uses the ESA Biosciences
maintenance-free palladium reference
electrode. The BDD is deposited
on a wafer, which is then cut to
the proper size and shape. The wafer
is coated with a conductive backing
layer. This electrode chip is then
placed into the 5040 Analytical cell
(ESA Biosciences).
Contact with the
electrode and sealing against a gasket
is made with the pin assembly, which
makes continuous contact with the
working electrode (Figure 3). The
cell is then connected to and is controlled
by a potentiostat such as the
Coulochem
III detector.
Applications of the
technology
Thiols and disulfides are widely recognized
as biologically important molecules.
For example, glutathione controls the
potential of living cells and is involved in
the metabolism of drugs. However, our
knowledge of the role of thiols has been
hampered by the difficulty in generating
reliable analytical data. A number of
methods are available, including those
found in numerous publications describing
the use of HPLC–ECD. Although
electrochemical detection is a viable and
desirable detection modality, it has not
gained widespread use because of the problems
when using carbon electrodes. Thiols
are reactive and readily adsorb; disulfides
require a high potential and suffer from a
poor signal-to-noise ratio. Contaminants
in the mobile phase and sample matrix,
unless painstakingly removed, cause rapid
degradation of response.

Figure 4 - A hydrodynamic voltammogram is created for each compound as part of the methods development process. With the BDD electrode, the optimal potential for detection of 11 thiols, disulfides, and thioethers was found to be +1400 mV, well beyond the maximum operating potential of typical carbon electrodes.
Choosing the optimal potential for
oxidation of thiols, disulfides, and
thioethers requires the generation of
a hydrodynamic voltammogram. This
is done by injecting a given amount
of the compounds onto the HPLC–ECD system at different applied
potentials. The signal is then plotted
as a function of the applied potential,
and the optimal potential (typically where the response plateaus) is chosen.
The optimum applied potential
for this analysis is found to be +1400
mV (versus Pd reference) (Figure 4).
This is well within the potential window
of the BDD electrode but too
high for conventional glassy carbon
working electrodes.
The BDD demonstrates very good stability
over time and multiple runs. The
response is typically stable for at least
65 hr for standards with 1.5% RSD
for reduced glutathione (GSH) and
6.5% RSD for glutathione disulfide
(GSSG). Routine analyses of plasma
extracts do not adversely affect the
performance. Typical chromatograms
are shown in Figure 5.
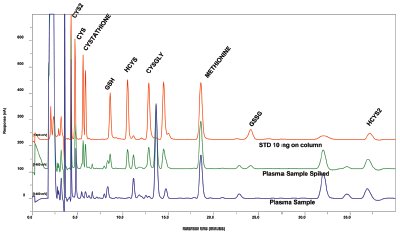
Figure 5 - Typical chromatograms for standards, plasma with standards added, and normal plasma. With the BDD electrode, routine analysis of plasma sample showed no deleterious effects on the signal.
Technology that makes HPLC–ECD more useful
Despite the high sensitivity and selectivity
of HPLC–ECD, it has been
limited in the scope of addressable
molecules by the working electrode
material. The BDD electrode broadens
the range of molecules now addressable
by this technique. Unlike other
working electrode materials, BDD
electrodes do not suffer from fouling
and do not degrade when subjected to
prolonged high-oxidation potentials.
BDD is a robust and rugged working
electrode material, well suited to the
measurement of thiols and disulfides.
References
- Acworth, I.N.; Bowers, M. An
Introduction to HPLC-Based Electrochemical
Detection: From Single
Electrode to Multi-Electrode Arrays.
In Coulometric Electrode Array Detectors
for HPLC; Acworth, I.N., Naoi,
M., Parvez, H., Parvez, S., Eds.; Progress
in HPLC-HPCE; Volume 6, VSP:
Utrecht,
1997; pp 3–50.
- Swain, G.M.; Ramesham, R. Anal.
Chem.1993, 65, 345.
- Xi, J.; Granger, M.; Chen, Q.; Strojek,
J.; Lister, T.; Swain, G. Anal. Chem. 1997, 69, 591A–7A.
- Tenne, R.; Patel, K.; Hashimoto, K.;
Fujishima, A. J. Electroanal. Chem. 1993, 347, 409–15.
- Rao, T.; Fujishima, A.; Angus, J. Historical
Survey of Diamond Electrodes in
Diamond Electrochemistry; Fujishima,
A., Einaga, Y., Rao, T., Tryk, D., Eds.;
Elsevier: Amsterdam, 2005; pp 1–10.
Mr. Waraska is Manager, In Vivo Voltammetry,
ESA Biosciences, Inc., 22 Alpha
Rd., Chelmsford, MA 01824, U.S.A.; tel.:
978-250-7083; fax: 978-250-7087; e-mail:
[email protected]. Dr. Acworth is Vice
President, HPLC Products and Services, ESA
Biosciences, Inc., and Adjunct Associate
Professor of Pharmacology, Massachusetts
College Pharmacy, Boston, MA, U.S.A.