Current research and development (R&D) that is focused on efficient energy conversion and environmental protection technologies relies heavily on advances in the development of new and improved functional nano-materials and nano-devices, such as catalysts, batteries, fuel cells, etc. A thorough understanding of the relationship between the nano-structure (shape, size, surface, interface, defects, etc.) of the nano-material system and its performance is vital for applied R&D.
High-resolution transmission electron microscopy (HRTEM) has clearly become an essential tool for studying nano-structured materials at the atomic scale. With more recent advances in electron optics, HRTEM is now able to detect the atomic structure in nano-materials with a resolution below 0.1 nm and with single atom sensitivity.
Standard TEM experiments are performed with the sample kept under high-vacuum conditions inside the microscope column. However, such conditions may be inadequate to investigate the active functional state of a structure whose properties depend on varying environmental (gas) conditions.1 In these cases, studies should preferably be performed in situ under exposure to an environment, such as a reactive gas environment, matching the conditions encountered during technical use of the nano-material. Dynamic, atomic-scale visualization of structural evolutions in situ under reactive (gas) environments directly addresses the environment-dependent structure and dynamics of the functional state of the nano-material and nano-device.2 This is a crucial R&D step, because generally there is no evidence that the dynamic state of the materials can be truly derived from postmortem (high-vacuum) examinations of the materials alone.
Differentially pumped environmental TEMs (ETEMs)3–10 are uniquely designed to permit some gas in the microscope’s specimen area while preserving the atomic-scale resolution of conventional TEM (see Figure 1).11 ETEM has proven to be an extremely powerful tool for applied research (e.g., different aspects in the field of heterogeneous catalysis research12), where it allows dynamic, in situ observations of catalysts in (near) operational conditions. Application of ETEM is an important complement to theoretical approaches as well as to the arsenal of established spectroscopic techniques (e.g., applied at synchrotron facilities) that average information over length scales considerably larger than the characteristic dimensions of the nanostructures themselves.
 Figure 1 – Titan ETEM G2 from FEI Company (Eindhoven, The Netherlands) and schematic drawing of microscope column and differential pumping scheme (see Ref. 11 for details).
Figure 1 – Titan ETEM G2 from FEI Company (Eindhoven, The Netherlands) and schematic drawing of microscope column and differential pumping scheme (see Ref. 11 for details).Some application examples utilizing in situ ETEM techniques are given below.
Studies of dynamic changes in nano-particles during redox processes
As mentioned above, dynamic observations of structural evolutions of functional nano-structures and nano-devices in their operating conditions are essential to understanding properties and function. Crozier et al.13 demonstrated the power of ETEM by exploring structural changes occurring in catalytic nano-particles during exposure to a reactive gas environment. In situ TEM studies have been applied to observe the dynamic changes taking place during redox reactions in ceria (CeO2) and ceria–zirconia (CexZr1-xO2) nanoparticles. Catalytic oxidation of hydrogen (H2) occurs via extraction of oxygen (O2) from the crystal lattice leading to a reduction of the catalyst itself:
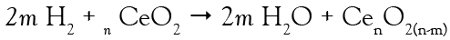
These reactions cause the catalyst to change composition and structure to accommodate the oxygen vacancies in the crystal lattice. A combination of different in situ applied TEM techniques (such as HRTEM, electron diffraction [ED], and electron energy loss spectroscopy [EELS]) have been applied to dynamically monitor the evolution of the structure and chemistry during the redox processes from room temperature (RT) up to 800 °C in a hydrogen (H2) gas atmosphere. For instance, in the case of pure ceria, a reversible phase transformation was found to take place at a temperature of 730 °C. The generated oxygen vacancies cause a structural change to a cubic lattice superstructure. Also, the ceria surface experienced structural transformations during reduction in hydrogen (H2). The (110) surface is initially constructed with a series of low-energy (111) nano-facets. Under strong reduction, the surface slowly transforms to a smooth (110) surface (see Figure 2).13
 Figure 2 – In situ environmental TEM studies of dynamic changes in cerium-based oxides nano-particles during redox processes. The in situ gas–solid reaction causes a change in composition and structure in a ceria crystal, dynamically observed by in situ HRTEM, electron diffraction (ED), and energy-loss spectra (EELS) recorded at 600 °C and at 730 °C in 0.5 Torr of hydrogen atmosphere (see Ref. 13 for details). (Reprinted with permission from
Figure 2 – In situ environmental TEM studies of dynamic changes in cerium-based oxides nano-particles during redox processes. The in situ gas–solid reaction causes a change in composition and structure in a ceria crystal, dynamically observed by in situ HRTEM, electron diffraction (ED), and energy-loss spectra (EELS) recorded at 600 °C and at 730 °C in 0.5 Torr of hydrogen atmosphere (see Ref. 13 for details). (Reprinted with permission from Ultramicroscopy
; copyright 2008, Elsevier.)Dynamic study of particle size evolution and particle size distributions
Assemblies of nano-particles dispersed on another material have turned out to be very efficient heterogeneous catalysts for a growing number of important applications. The catalytic properties depend not only on the choice of combination of materials, but also on the stable shape, size, and distribution of the nano-particles on the surface of the support. However, in many cases in which heterogeneous catalyst nano-particles are in their reaction environments at elevated temperatures, there is a change in particle size due to sintering, Ostwald ripening, etc., and particle distribution (e.g., see Refs. 14 and 15) that will eventually deactivate the catalyst.
As a recent example of research utilizing an ETEM, Benavidez et al.16 heated palladium nano-particles (using a characterizing heating cycle) in hydrogen (H2) inside a microscope to study the role of the reactive gas environment. A series of in situ TEM images taken dynamically during part of such a heating sequence are shown in Figure 3. It appears that when particles are in close proximity, they move close to one other and coalesce. The series in Figure 3 show that some adjacent particles eventually merge to form a single, larger particle. In this case, the coalescence of two particles seems to be driven by atomic-scale migration: the process of merging is triggered by formation of a neck or bridge that then fills in via atoms migrating to the reentrant surface. Due to its size and shape change, this resulting larger particle might exhibit a different catalytic property, which is not beneficial for the desired chemical conversion process.16
 Figure 3 – Dynamic in situ TEM study of particle size evolution and distribution in supported metal catalysts in a gas environment. Images of a time series of the same region of palladium nano-particles on a carbon film heated to 600 °C in a 3 mbar gas atmosphere of 5% hydrogen/argon. The images show the formation of a neck between two large, adjacent particles and their eventual coalescence after more than 6 min (see Ref. 16 for details). (Reprinted with permission from
Figure 3 – Dynamic in situ TEM study of particle size evolution and distribution in supported metal catalysts in a gas environment. Images of a time series of the same region of palladium nano-particles on a carbon film heated to 600 °C in a 3 mbar gas atmosphere of 5% hydrogen/argon. The images show the formation of a neck between two large, adjacent particles and their eventual coalescence after more than 6 min (see Ref. 16 for details). (Reprinted with permission from ACS Catalysis
, copyright 2012, American Chemical Society.)Reliability studies of solid oxide fuel cell (SOFC) nano-material
In the future, energy supply may be based more on sources such as hydrogen (H2) than on the continuous use of fossil fuel. Depending on the source of H2, this would also lead to a reduction in the emission of greenhouse gases. The chemical reaction of H2 with oxygen (O2):
would provide the energy for electricity and transportation. One of the key technologies here, which is the focus of ongoing R&D work, is the fuel cell (FC). FCs are electrochemical devices for the conversion of chemical to electrical energy. They are efficient, scaleable, have a rapid response to load changes, and are suitable for remote usage.
Solid oxide fuel cells are a class of FCs that use a solid oxide material as the electrolyte. Jeangros et al.17 performed in situ ETEM studies on SOFC anode material mimicking multiple FC operation cycles to study the redox stability (Figure 4). Here, the SOFC anode is made of porous ceramic–metal composite yttria stabilized zirconia (YSZ) and nickel (Ni). The results reveal that the transfer of oxygen from NiO to YSZ triggers the reduction reaction. During Ni reoxidation, the creation of a porous structure, due to mass transport, accounts for the redox instability of the Ni-based anode. Both the expansion of NiO during a redox cycle and the presence of stress in the YSZ grains are observed directly. In addition to providing an understanding of the anode redox degradation, the observations are used to propose an alternative anode design for improved redox tolerance.17
 Figure 4 – In situ TEM study of the redox cycle of a solid oxide fuel cell anode. The in situ TEM image series show a redox cycle of a NiO-YSZ-based SOFC anode (in situ reduction in 1.4 mbar H2 and subsequent reoxidation in 3.2 mbar O2. Structural changes (such as nano-porosity and cracks) develop during in situ redox cycles mimicking the operational cycles of an actual FC (see Ref. 17 for details). (Reprinted with permission from
Figure 4 – In situ TEM study of the redox cycle of a solid oxide fuel cell anode. The in situ TEM image series show a redox cycle of a NiO-YSZ-based SOFC anode (in situ reduction in 1.4 mbar H2 and subsequent reoxidation in 3.2 mbar O2. Structural changes (such as nano-porosity and cracks) develop during in situ redox cycles mimicking the operational cycles of an actual FC (see Ref. 17 for details). (Reprinted with permission from Acta Materialia
, copyright 2010, Elsevier.)Atomic-scale observations of surface oxidation
Surface oxidation, such as on metals, plays an important role in different technologies, from causing serious long-term materials stability issues to providing a defined beneficial protection layer. Areas of research include controlling surface morphology and manipulating initial oxidation steps to affect the ongoing kinetics. Zhou et al.18,19 recently reported dynamic atomic-resolution ETEM observations of in situ oxidation on copper (Cu) surfaces.
The structure difference between the idealized crystal surfaces and real surfaces is known as the structure gap and has been a major obstacle hindering understanding of the (microscopic) processes controlling the oxidation of surfaces. The observed realistic Cu surfaces are composed of flat terraces and step edges. Oxidation occurs via layer-to-island growth of Cu2O on flat terraces, with Cu adatoms evaporating from steps and diffusing across the terraces (Figure 5). This process can be regarded as deposition of a solid oxide from a mixed phase of Cu and O thermally diffusing across the surface that is rather different from the long-held oxidation mechanism of the solid–solid transformation. The presence of surface steps can promote the development of a flat metal–oxide interface by kinetically suppressing the solid–solid transformation of oxide formation. Control of the surface morphology therefore allows manipulation of the oxidation behavior of metals.19
 Figure 5 – Atomic-scale visualization of a metal surface oxidation. In situ TEM observation of monolayer growth of Cu2 on a Cu(110) surface during in situ oxidation at 350 °C in 0.005 Torr of oxygen gas atmosphere (see Ref. 18 for details). (Reprinted with permission from
Figure 5 – Atomic-scale visualization of a metal surface oxidation. In situ TEM observation of monolayer growth of Cu2 on a Cu(110) surface during in situ oxidation at 350 °C in 0.005 Torr of oxygen gas atmosphere (see Ref. 18 for details). (Reprinted with permission from Microscopy and Microanalysis
, copyright 2012, Cambridge University Press.)Conclusion
The above application examples and others12 (see, also, a reference list at FEI.com/ETEM) demonstrate that a differentially pumped ETEM is a powerful tool for applied research and development on functional nano-structures (such as catalysts, batteries, and fuel cells) that requires exposure to an operational/reactive gas environment. ETEM provides exclusive data (images, movies, spectra, and more) from these dynamic in situ processes at the atomic scale.
The number of centers worldwide that use FEI ETEM techniques as part of their ongoing research continues to grow. Many of these centers also operate as user facilities, thereby opening opportunities for researchers facing similar R&D challenges to explore the ETEM capabilities in collaboration with specialists at one of these centers.
References
- Ertl, G.; Knözinger, H. et al., Eds. Handbook of Heterogeneous Catalysis, 2nd ed.; VCH-Wiley: Weinheim, 2008.
- Yoshida, H.; Kuwauchi, Y. et al. Visualizing gas molecules interacting with supported nanoparticulate catalysts at reaction conditions. Science 2012, 335(6066), 317–19.
- Boyes, E.D.; Gai, P.L. Environmental high resolution electron microscopy and applications in chemical science. Ultramicroscopy 1997, 67, 219–32.
- Sharma, R.; Weiss, K. Development of a TEM to study in situ structural and chemical changes at an atomic level during gas–solid interactions at elevated temperatures. Microsc. Res. Tech. 1998, 42, 270–80.
- Hansen, T.W.; Wagner, J.B. et al. Atomic-resolution in situ transmission electron microscopy of a promoter of a heterogeneous catalyst. Science 2001, 294(5546), 1508–10.
- Gai, P.L. Developments in in situ environmental cell high-resolution electron microscopy and applications to catalysis. Topics Catal. 2002, 21, 161–73.
- Hansen, P.L.; Wagner, J.B. et al. Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science 2002, 295(5562), 2053–5.
- Sharma, R.; Crozier, P.A. Environmental Transmission Electron Microscopy in Nanotechnology. In: Handbook of Microscopy for Nanotechnology; Yao, N.; Wang, Z.L., Eds.; Kluwer Academic Publishers: New York, NY, 2005; pp 531–65.
- Hansen, P.L.; Helveg, S. et al. Atomic-scale imaging of supported metal nanocluster catalysts in the working state. Adv. Catal. 2006, 50, 77–94.
- Hansen, T.W.; Wagner, J.B. et al. Aberration corrected and monochromated environmental transmission electron microscopy: challenges and prospects for materials science. Mat. Sci. Technol. 2010, 26(11), 1338–44.
- Jinschek, J.R.; Helveg, S. Image resolution and sensitivity in an environmental transmission electron microscope. Micron2012, 43(11), 1156–68.
- Jinschek, J.R. Atomic scale structure–function relationship of heterogeneous catalysts: investigation of gas–solid interactions by ETEM. Microscopy and Analysis 2012, 26(7), 5–10.
- Crozier, P.A.; Wang R. et al. In situ environmental TEM studies of dynamic changes in cerium-based oxides nanoparticles during redox processes. Ultramicroscopy 2008, 108, 1432–40.
- Simonsen, S.B.; Chorkendorff, I. et al. Direct observations of oxygen-induced platinum nanoparticle ripening studied by in situ TEM. J. Am. Chem. Soc. 2010, 132, 7968–75.
- Simonsen, S.B.; Chorkendorff, I. et al. Ostwald ripening in a Pt/SiO2 model catalyst studied by in situ TEM. J. Catalysis 2011, 281, 147–55.
- Benavidez, A.D.; Kovarik, L. et al. Environmental transmission electron microscopy study of the origins of anomalous particle size distributions in supported metal catalysts. ACS Catalysis 2012, 2, 2349—56.
- Jeangros, Q.; Faes, A. et al. In situ redox cycle of a nickel–YSZ fuel cell anode in an environmental transmission electron microscope. Acta Materialia 2010, 58, 4578–89.
- Luo, L.; Li, L. et al. Atomic-scale visualization of the oxidation of Cu surfaces via in situ environmental TEM. Microsc. Microanal. 2012, 18(Suppl. 2), 1122–3.
- Zhou, G.; Luo, L. et al. Step-edge induced oxide growth during the oxidation of Cu surfaces. Phys. Rev. Lett. 2012, 109, 235502.
Dr. Joerg R. Jinschek is Application Scientist and Product Marketing Manager in the Materials Science Business Unit, FEI Company, Achtseweg Noord 5, Eindhoven, The Netherlands; tel.: +31 40 23 56000; e-mail: [email protected]. The author acknowledges the outstanding scientific work and contributions of the researchers and institutions that drive research in the field of in situ environmental studies utilizing ETEM microscopes. The author wishes to thank these researchers and the entire ETEM community for the education, encouragement, and fruitful discussions. Also, he gratefully acknowledges the continuous support of FEI’s ETEM team and Materials Science Business Unit (FEI.com/ETEM).