In the R&D laboratories of universities and the pharmaceutical industry, microwave synthesis is becoming increasingly popular. Especially for method development and the optimization of chemical transformations, the use of dedicated industrial microwave reactors is extremely beneficial and, most importantly, saves time. The system described in this article allows for reactions under enhanced conditions and thus significantly extends the scope of microwave chemistry.

Figure 1 - Monowave 300 microwave reactor.
The Monowave™ 300 (Anton Paar GmbH, Graz, Austria) (Figure 1) is a monomode microwave synthesis reactor for chemical transformations under sealed vessel conditions. With a powerful 850-W magnetron and specially designed microwave applicator, very high field density can be attained to achieve optimum heating rates for various solvents in any volume (see Figure 2). In addition to microwave absorbers such as alcohols, N-methyl pyrrolidinone (NMP), and dimethylformamide (DMF), weak-absorbing solvents such as acetonitrile and tetrahydrofuran (THF) can be heated rapidly far above their boiling points. For nearly microwave-transparent compounds such as toluene and dioxane, a special 10-mL silicon carbide (SiC) vessel is available.1,2
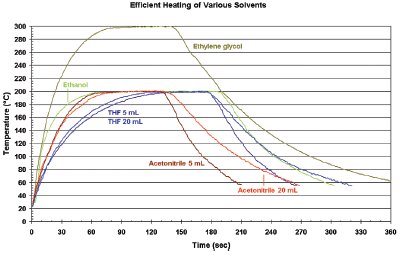
Figure 2 - Heating profiles of common solvents.
The system offers high microwave output power in conjunction with operating limits of 300 °C and 30 bar (435 psi). It utilizes reusable reaction vials, constructed of standard borosilicate glass, that are sealed with durable snap caps and PTFE-coated silicone septa. Both 10-mL and 30-mL glass vials are available for reaction volumes of 2–6 and 6–20 mL, respectively. The SiC vessel can accommodate volumes well below 2 mL. The compact system provides significant scaleability for R&D purposes, since syntheses in gram scale can be carried out in order to generate products in sufficient quantity for analysis and total characterization.

Figure 3 - MAS 24 autosampler.
With the optional MAS 24 autosampler (Figure 3), up to 24 vials of any type can be queued and processed at the same time. Because the autosampler is mounted directly on top of the Monowave 300, no additional laboratory space is required.
Due to the high field density, the heating profiles of a solvent (or mixture) are practically identical, regardless of the applied volume (see Figure 2). Therefore, any optimized method can remain unchanged in different scale. The extended pressure limit is particularly important because it allows the use of low boiling solvents at much higher temperatures (e.g., ethanol at 200 °C, tetrahydrofuran at 225 °C, acetonitrile at 240 °C, dichloroethane at 245 °C, and toluene at 290 °C), which was not possible with previous monomode microwave reactors. Thus, chemical transformation efficiency will increase since reaction times can likely be reduced, even more at higher temperatures.
Sensor technology

Figure 4 - Monowave 300 swiveling cover.
Pressure sensing is accomplished by a hydraulic sensor integrated into the locking, swiveling cover of the instrument (see Figure 4). When the cover closes, a pneumatic system provides pressure-tight sealing of the vials. The software controller enables reactions up to 30 bar (435 psi); at 35 bar (508 psi) the process is automatically terminated and the pneumatic system opens to safely release the overpressure. The same action occurs at the regular end of a run if remaining overpressure is present after cooling. Thus, if the swiveling cover opens, the vials are guaranteed to be pressure-less, as at the beginning of the experiment. Since the pressure measurement is a noninvasive technique, the septa will not be penetrated and can be reused several times without any problem.
The most important control parameter for reproducibility of chemical transformations is the reaction temperature. Traditionally, monomode reactors provided only one mode of temperature sensing (mainly external by IR); the Monowave 300 offers dual temperature measurement. A standard, integrated IR sensor measures the temperature at the curve of the vessel. The system’s software algorithm prevents overshooting of the target temperature to ensure accurate reaction control.
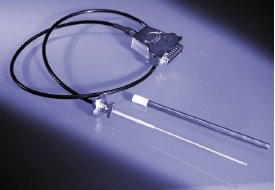
Figure 5 - Ruby thermometer.
For even more accurate temperature measurement, an optional immersing fiber optic thermometer can be used (Figure 5). This tool allows precise control of reaction conditions, even when the highest heating rates are reached. Again, the software algorithm ensures that the target temperature will not be exceeded. The sophisticated state-of-the-art system is based on a ruby crystal excited by a light duct that causes light emission (photoluminescence) of the ruby. The decay time of the photoluminescence is measured since it is directly related to the temperature.
The ruby thermometer is a simple plug-and-play tool. Once connected to the serial port at the rear of the instrument, it is ready to use. The tool enters an opening in the swiveling cover and is fixed in place with a simple bayonet lock.
Another option is that the values of both sensors can be displayed simultaneously. The user can set the IR or fiber optic sensor to work as master or slave for temperature control. The immersing temperature probe is important, especially for general research in academic laboratories, because it enables the exact determination of the current reaction temperature.1,3 Measuring the internal temperature increases the quality of optimized protocols and enhances the level of reproducibility. The better elaborated the method and the more that is known about the real reaction temperature, the easier it is the transfer the method to another device.
Conclusion

Figure 6 - Touchscreen operation.
Out of the box, the Monowave 300 is ready to use in minutes. Utilizing a large, 8.4-in. touchscreen (Figure 6), all of the necessary handling steps are performed with the clearly structured, intuitive firmware package. From simple experiments to multistep processes, all methods can be programmed quickly.
The most important parameters, i.e., target temperature and hold time, can be modified during experiment processing via immediate, one-click editing. Other menus of the user interface are accessible at any time, with the experimental progress thoroughly controlled and recorded in the background. With the push of a button, GxP-compliant experiment reports can be generated, exported, and printed in PDF format.
Up to 1000 methods can be stored on the instrument and recalled for repeat processing. Easy access to the required files is supplied by sorting filters according to user, date, experiment name, etc. Data export (.pdf, .xls, or .txt format) is via USB or Ethernet connection.
With all of these advantages, namely, performance, reliability, and sophisticated technology, the Monowave 300 microwave synthesis reactor is a valuable tool for the modern research laboratory. Features include high experiment reproducibility as well as the scaleability and transferability of optimized methods to larger microwave systems such as the Synthos 3000 parallel microwave synthesis platform or Masterwave Benchtop Reactor for kilolab approach (both from Anton Paar). Although the major areas of application are universities and the pharmaceutical industry, the Monowave 300 can serve other areas in microwave chemistry, such as materials science (zeolites, nanostructures, etc.) and polymer synthesis.
References
- Obermayer, D.; Guttmann, B.; Kappe, C.O. Angew. Chem. Int. Ed. 2009, 8321–4.
- Guttmann, B.; Obermayer, D.; Reichard, B.; Prekodravac, B.; Irfan, M.; Kremsner, J.M.; Kappe, C.O. Chem. Eur. J.2010, 12,182–94.
- Obermayer, D.; Kappe, C.O. Org. Biomol. Chem. 2010, 114–21.
Dr. Stadler is Product Manager, Microwave Synthesis, Anton Paar GmbH, Anton-Paar-Str. 20, 8054 Graz, Austria; tel.: +43 (0) 316 257 3560; fax: +43 (0) 316 257 9356; e-mail: [email protected].