A brief discussion about dissolution is important to understand the relevance of automation to this field. Dissolution testing is a critical test for measuring the performance of a drug product. In the last few years, the importance of the dissolution test has increased substantially. Dissolution testing plays several key roles in the pharmaceutical industry. Primarily, the test is a quality control tool that measures change on stability of the drug product. The relevant changes in the drug product are caused by temperature, humidity, photosensitivity, etc. The test is also important for formulation development. During development of the drug products, the formulators use the dissolution test to discriminate between variants of the drug product. Furthermore, once in vivo data have been established for the drug product, a correlation between in vivo/in vitro is attempted.

Figure 1 - Apparatus 1 and 2 and pH regions of the body.
The dissolution procedure is composed of a dissolution apparatus, the dissolution medium, and the test conditions that provide a method with discriminatory and robust abilities. There are two main dissolution apparatuses that are widely used: apparatus 1 (baskets) and apparatus 2 (paddles) (see Figure 1).
According to the FDA’s Guidance for Industry (Dissolution Testing of Immediate Release Solid Oral Dosage Forms), the Agency recommends use of dissolution medium with a pH range of 1.2–6.8. This range represents the biological digestive region made up of the stomach (dissolution medium using 0.1N or 0.01N HCl), duodenum (dissolution medium using pH 4.5 acetate buffer), and intestinal region (dissolution medium using pH 6.8 phosphate buffer) (see Figure 1 for more detail). The dissolution medium is held at 37.0 ± 0.5 °C representing the biological body temperature. The FDA further recommends that dissolution testing be carried out under mild conditions—basket method at 100 rpm or paddle method at 50–75 rpm. Once the tablet or capsule is introduced into the dissolution medium, the analyst has to collect aliquots of samples at different time points depending on the run time. The run times can vary from 30 min to several hours. As can be seen, the entire dissolution setup and sample collection are very labor-intensive processes.
Why is there a need to automate? The current system is not broken, so why fix it? There are many reasons the industry has to automate. First, in a competitive global market, the pharmaceutical industry finds itself trying to test more samples in a fraction of the time. Laboratory managers are being asked to increase quantity while increasing quality. The faster the tests can be completed, the faster the needed drugs can make it to market. Second, as previously pointed out, the dissolution process is very labor-intensive. With so many steps involved, the test can suffer from analyst-to-analyst variability. Most of the activities required for dissolution testing are routine tasks. These include filling vessels, cleaning vessels, emptying vessels, sampling, and so forth. These laborious and routine tasks lend themselves very well to automation. Third, dissolution testing is a highly regulated activity. In fact, during development, most of the questions asked by health agencies such as the FDA and EU Health Authority pertain to dissolution testing.
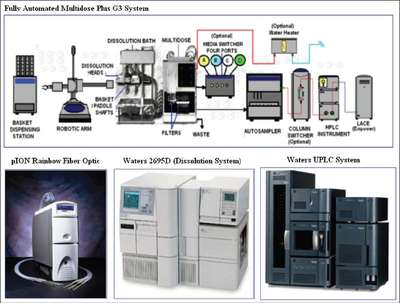
Figure 2 - Dissolution technologies available.
With so much emphasis placed on dissolution testing in the pharmaceutical industry, automation becomes a key element in ensuring consistency, quality, and ease of use. Several commercially available automated technologies can be utilized in the dissolution laboratory. These include the Multidose® G3 Plus system (Caliper Life Sciences, Hopkinton, MA), UV-VIS Fiber Optic Dissolution System (pION Inc., Woburn, MA), Waters 2695D (Waters Corp., Milford, MA), and UPLC® system (Waters Corp.) (see Figure 2 for an illustration of these technologies). This paper will focus on application of the Multidose G3 Plus system in the laboratory.
The system is composed of a basket dispensing station; a robotic arm; a dissolution bath (Varian Inc., Palo Alto, CA); the main unit; the media switcher, which has four separate media attachments; an XYZ autosampler (optional) or Multifill collection rack; a column switcher (optional); and an HPLC system (optional) for real-time data collection. The system can also run in a semiautomated mode that does not contain the XYZ autosampler or HPLC system. Moreover, the system’s Multifill collection rack is able to collect samples for later analysis. The automated dissolution system can run eight dissolutions during one batch process. This allows the laboratory to run dissolutions during the daytime and set up the system to run during the evening. With this ability to run day and night, large numbers of tests can be generated per year. In fact, over the course of the last few years, the number of samples submitted for dissolution testing grew at a rate of 10–20%. Since the resources remained constant during these years, the automated systems were able to keep up with the high demand.
Table 1 - Number of dissolutions (manual vs automated) vs time

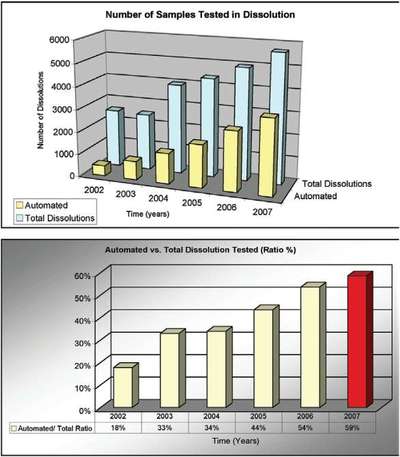
Figure 3 - Number of dissolutions vs time (years).
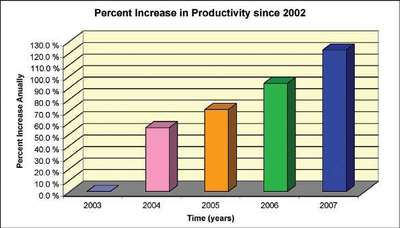
Figure 4 - Productivity gains.
Table 2 - Productivity gains

Productivity gains
Table 1 and Figure 3 depict the number of dissolutions tested during the last several years. In 2007, a total of 5683 dissolutions were tested, and 59% of these tests were carried out using the automated dissolution systems. These numbers were achieved using four automated dissolution systems. As the demand for dissolution testing continues to grow, there is an expectation that the percentage of samples tested using an automated system will grow as well.
In addition, from a productivity standpoint, the automated dissolution systems do a very good job (see Figure 4 and Table 2). In fact, since 2005, the company was able to increase productivity at an annual rate of not less than 10% with the same resources. Since the demand for samples to be tested and quality increased, the automated systems were able to counter these high demands. In 2007, 5683 dissolution tests were conducted in the East Hanover, NJ laboratory. This means about 34,000 tablet/capsule drops were made over the course of the year. Comparing these numbers to 2006, the increase in productivity reached a staggering 15%, again with the same resources. The quality of the work improved during these years. The automated systems were able to perform the tests consistently. There is no analyst-to-analyst variability involved. Systems are in place to mitigate and eliminate mechanical errors associated from the systems.