Monday, February 18, 2008
Acceptability of food by consumers is highly governed by its flavor (aroma and taste) perception. As has been reviewed by many authors, flavor release depends on the physicochemical composition of the food matrix.1 The presence of proteins often causes a decrease in the volatility of aroma compounds that are able to form reversible or irreversible binding.2 The strength of the interactions is highly dependent on the nature of both the aroma and the protein.3 Fat has a significant effect on the partition of volatile compounds between the food and the air phase, and this effect is greater than that of proteins and thickeners.4 The volatility of aroma compounds in fat is highly dependent on the chain length and degree of nonsaturation of hydrocarbon chains.5 Fat reduction has an effect on flavor perception not only due to a lower aroma release but also due to a change in viscosity and/or tastant release.6
Food flavor, taste, and texture sensations are perceived over time during consumption, and intensity of flavor perception can change over time. The temperature of the product will, after time, equilibrate with the mouth temperature. Physical manipulations such as tongue movements, mastication, and salivary dilution will affect the product and its sensory characteristics over time.7 The classical methods of descriptive sensory analysis do not take into account this temporal dimension. For that purpose, dynamic methods such as time intensity were developed.8
This paper discusses a study on the influence of the nature of fat and protein added to model food emulsions, aroma release followed by solid-phase microextraction (SPME) measurements, and flavor perception followed by time-intensity sensory analysis.
Experimental
Preparation of the food model emulsions
Emulsions were composed of 3% milk proteins, 9% fat, and 0.5% emulsifier. Two fats were used: one partially hydrogenated palm (HP) kernel oil and an anhydrous milk fat (AMF), which was less polar. These fats were provided by Aarhus Oliefabrik A/S (Malmö, Sweden) and Lactalis industrie: Besnier-Bridel-Aliment (Bourgbarre, France), respectively. Two batches of protein were used, differing in their composition in whey proteins: a milk powder (MP) (0% fat) (Besnier-Bridel-Aliment), and a mixture of whey protein (WP) concentrate (Lacprodan DI 9224) and β-lactoglobulin (Protarmor 865, Armorproteine).
Table 1 - Physicochemical and sensory parameters of aroma compounds
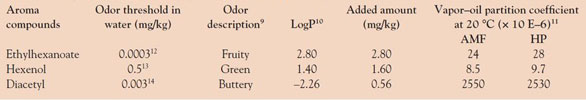
The model food emulsions were flavored with three aroma compounds that have different hydrophobicity, hexenol, diacetyl, and ethylhexanoate (Table 1), after dissolution in propylene glycol. These compounds were obtained from flavor suppliers (Food Ingredients Specialities [FIS], York, U.K.).
SPME coupled with GC-MS (SIM mode) analysis
- SPME sampling. Food model emulsions (5 g) were placed in 20-mL vials and allowed to equilibrate at different temperatures (10, 15, 20, and 25 °C). A 65-μm SPME fiber, polydimethyl siloxane/divinylbenzene (PDMS/DVB) (Supelco, Bellefonte, PA), was used for volatile compound sampling, using the methodology already described.3 Volatile compounds were desorbed by inserting the fiber into the GC injector, set at 250 °C for 10 min, 1 min for desorption (purge off), and 9 min for cleaning (purge on). All SPME operations were automated using an MPS2 multipurpose sampler (Gerstel Applications, Brielle, The Netherlands). Experiments were done in triplicate.
- GC-MSanalysis. An HP 6890 GC equipped with a split/splitless injector coupled with a 5970 mass selective detector (Hewlett Packard, Palo Alto, CA) was used. A DB-Wax fused-silica capillary column (50 m, 0.32 mm i.d., 1 μm film thickness) (J&W Scientific, Folsom, CA) was employed. The carrier gas was helium (35 cm/sec). GC oven heating was started at 50 °C, and was increased to 220 °C at a rate of 5 °C/min. The total analysis time was 39 min. The injector was constantly maintained at 250 °C. The mass spectrometer was operated in the mass range 29–300 at a scan rate of 1.89 sec/scan. Quantification was performed in selective ion monitoring (SIM) mode. The selected and specific ions were 43 for diacetyl, 101 for ethylhexanoate, and 82 for hexenol.