Intrabiopsy and intraoperative cancer detection has been a major challenge for early diagnosis of cancer and accurate determination of surgical margin. Using common light microscopy, cancer and nerve tissues are not usually distinguishable from normal tissue structures, posing the main obstacle to the identification of different tissue types. As a result, definitive diagnosis is only accessible after tissue section and staining, a lengthy process that is always incompatible with the timeline of surgical interventions. Targeting this problem, substantial research efforts have been allocated to provide real-time diagnostic capability.1 Within all imaging modalities, optical methods are normally preferred, given their cellular resolution, a condition precedent to effective diagnostic judgment. This article describes a novel molecular diagnostic strategy using label-free modality to handle the challenges of intraoperative imaging by combining the coherent anti-Stokes Raman scattering (CARS) optical imaging technique with a computerized image analysis system.
A diagnostic imaging system defines and characterizes the imaged regions with regard to various tissue structures. To reach a high level of accuracy and specificity, dyes and molecular contrast agent are commonly used to assist optical techniques to highlight certain cell types or cellular components. However, such approaches are usually limited by the fact that the chemical agents face a lengthy and expensive FDA approval process and, unfortunately, with low success rate. For translational purposes, label-free techniques provide a more promising route to clinics. CARS, which is representative of such techniques, utilizes intrinsic vibrations of chemical bonds to generate optical contrast,2 a premier feature that effectively eliminates the need for chemical dyes, thus offering substantial promise for clinical applications.
Coherent anti-Stokes Raman scattering process
In a CARS process, a pump field (ωp), Stokes field (ωs), and probe field (ωp’—typically the same as ωp)— interact with the samples through a four-wave mixing process.2 When the frequency difference, ωp – ωs (beating frequency), is in resonance with a molecular vibration, an enhanced signal at the anti-Stokes frequency—ωas = ωp – ωp + ωp’—is generated in a direction determined by the phase-matching conditions. The major advantage of CARS is that the signal yield is much higher, typically several orders of magnitude, than the signal yield obtained through the spontaneous Raman scattering process.3 Since no natural or artificial fluorescent probes are required, CARS effectively avoids the toxicity, photobleaching, and artifacts associated with the staining process. By tuning the beating frequency, CARS provides chemically selective excitation of characteristic vibrational resonances, allowing imaging of particular chemical structures at will. Combined with a fast scanning platform, this feature allows imaging of tissue and cell structures on a 3-D scale in real time.

Figure 1 - CARS and corresponding H&E images of normal and cancerous human prostate glands. (Figure 1d-f reproduced with permission from Ref. 6.)
Figure 1 illustrates CARS images of human normal and cancerous prostate glands and their standard hematoxylin and eosin stain (H&E) results from similar fields of view from the same specimen. Normal gland possesses well-oriented cellular structures, while cancerous gland shows clustered and enlarged cells with cribriform growth pattern. Since the prostate has a poorly defined capsule, identification of these glandular structures will potentially allow better separation of cancerous prostate tissue from periprostatic structures for identification of surgical margins.
In addition to cellular resolution, CARS allows 3-D imaging through optical sectioning. As mentioned above, the intensity of CARS depends nonlinearly on two incident intensities; therefore CARS signals are tightly restricted to a very thin (around 1 μm) focal plane. By acquiring images from different depths at a predetermined sampling interval (called a Z-stack), 3-D reconstruction is feasible.
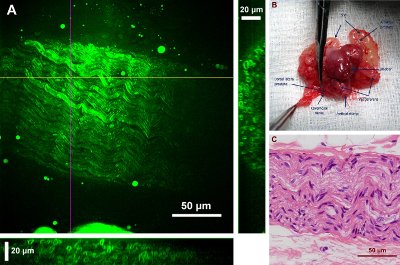
Figure 2 - CARS image of a rat cavernous nerve and the anatomy location as well as the H&E stained structure of the same nerve.
Figure 2 shows a 2-D projection of a rat cavernous nerve (CN) using a 27-μm-deep Z-stack and its corresponding anatomical as well as H&E result. CNs are postganglionic parasympathetic nerves that facilitate penile erection. Because of the lack of precise intra-operative nerve identification, these nerves are usually damaged during prostatectomy, causing the major challenges of postsurgical recovery of erection function. However, when imaged with CARS, strong signals originate from lipid-rich myelin sheaths on the outer surface of the nerve fibers. Compared to adjacent muscle and glandular tissues, CNs and their surrounding fat cells have much brighter CARS emissions. This feature offers a feasible strategy to quickly differentiate CNs from prostate and other periprostatic structures prior to surgical incisions.
Use of CARS in clinical diagnostics
CARS technology offers a substantial opportunity for real-time imaging of tissue of concern for definitive diagnosis and treatment. However, two common problems can impede development of such devices for clinical use. First, for clinical diagnosis, an effective imaging and analytical platform should be developed for accurate evaluation of imaging data. Second, the imaging system needs to be miniaturized to a size compatible with clinical intervention.
Currently, in order to accurately delineate the type of lesions, pathologists routinely stain biopsy tissue to examine changes in such cellular and histologic features as cell size, cell–cell distance, and formation of fibrous structures.4 However, while this method is subject to interobserver variations, the CARS technique provides high-resolution images that can clearly detect these features. Therefore, to address the first problem, the authors have successfully combined this label-free imaging technique with a pattern recognition method whereby such images were used as the basis for quantitative classification of these cellular features in a way that would lead to a differential analysis of tissue of concern. The platform developed has been tested successfully on lung cancer, prostate cancer, and breast cancer,5–7 three major types of cancer in the U.S. Figure 3 shows a 3-D spatial separation of normal, benign, and cancer samples using partial least squares regression (PLSR) analysis. Morphological characteristics—including nuclear size, cell volume, and cell–cell distance—were measured from nuclear segmentation of CARS images and used in this analysis for faithful interpretation of the disease type in alliance with known pathological criteria.
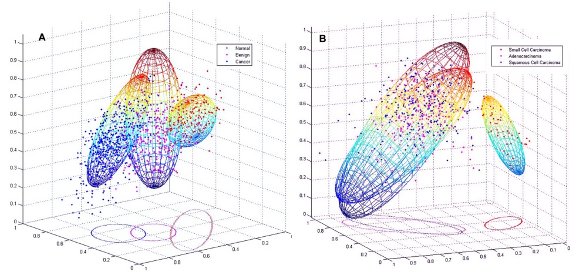
Figure 3 - Spatial distributions of a) cancer, normal, and benign cells; and b) cancer subtypes using PLSR analysis for computerized separation of different tissue lesions. (Reproduced with permission from Ref. 5.)
The second problem has attracted much research attention. To date, building a fiber probe is an unresolved problem facing CARS microendoscope development due to poor efficiency in laser delivery and signal collection, as well as difficulties in minimization of nonlinear effects and miniaturization of optics and scanning mechanisms. To address these problems, the Wong laboratory devised a fiber probe that replaces conventional single-model fibers with multimode fibers to improve efficiency of laser delivery and signal collection while relieving nonlinear effects,8 and adapts a polarization-based four-wave-mixing (FWM) suppression strategy to reduce FWM background.9 This raster-scan CARS microendoscope prototype was constructed as a proof-of-concept strategy for miniaturization of the CARS system. Specifically, using a polarization-based scheme in the optical fiber for delivery of both excitation lasers and reflected CARS signals, the FWM background signal was identified and suppressed.

Figure 4 - CARS images of 10-μm PEBs. a) Parallel-polarized pump and Stokes beams without the DWW insertion, b) orthogonal-polarized pump and Stokes beams without the DWW insertion, c) orthogonal-polarized pump and Stokes beams with the DWW insertion, d) intensity profiles along vertical green lines in Figure a–c, e) CARS spectrum of PEBs at pump (800 nm–826 nm) and Stokes beams (1064 nm), and f) mouse skin tissue. Scale bar is 10 μm. (Reproduced with permission from Ref. 10.)
Figure 4 shows the use of this prototype for imaging 10-μm polystyrene beads (PEB) as a standard test sample. Laser delivery can be well controlled using a dual-wavelength waveplate (DWW) for suppression of FWM signals. Imaging of mouse skin tissues was also demonstrated as a prestep for future imaging on patient samples.
Quantitative imaging and CARS-based microendoscopy
In summary, optical imaging technologies offer revolutionary changes aimed at improving health care and advancing biomedical research. Bypassing the lengthy FDA approval process commonly required for label-based approaches using exogenous dyes, label-free methods provide a facilitated route for building novel imaging instrumentation for human disease diagnosis and treatment. As such a method, CARS has shown substantial potential for intraoperative applications by delineating pathologically related cell morphology information.
To fully utilize its diagnostic value, a quantitative imaging platform was developed. The goal is to provide accurate evaluation of imaging data in a repeatable manner, promoting the translation of this technology for patient care. Finally, the development of CARS-based microendoscopy9–13 has the potential to change the current practice of intrabiopsy and intraoperative imaging, and thus enables in vivo molecular diagnosis at single cell resolution while alleviating patients suffering and medical expense.
References
- Nguyen, Q.T.; Olson, E.S. et al. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc. Natl. Acad. Sci. USA 2010, 107(9), 4317–22.
- Cheng, J.-X.; Xie, X.S. Coherent anti-Stokes Raman scattering microscopy: instrumentation, theory, and applications. J. Phys. Chem. B 2004, 108(3), 827–40.
- Djakera, N.; Lennea, P.-F. et al. Coherent anti-Stokes Raman scattering microscopy (CARS): instrumentation and applications. Nucl. Instr. Meth. Physics Res. A 2007, 571(1–2), 177–81.
- Kumar, V.; Abbas, A.K. et al., Eds. Pathologic Basis of Disease; Saunders: Philadelphia, PA, 8th ed.; 2009.
- Gao, L.; Li, F. et al. On-the-spot lung cancer differential diagnosis by label-free, molecular vibrational imaging and knowledge-based classification. J. Biomed. Opt. 2011, 16(9), 096004.
- Gao, L.; Zhou, H. et al. Label-free high-resolution imaging of prostate glands and cavernous nerves using coherent anti-Stokes Raman scattering microscopy. Biomed. Opt. Express 2011, 2(4), 915–26.
- Yang, Y.; Li, F. et al. Differential diagnosis of breast cancer using quantitative, label-free and molecular vibrational imaging. Biomed. Opt. Express 2011, 2(8), 2160–74.
- Wang, Z.; Yang, Y. et al. Delivery of picosecond lasers in multimode fibers for coherent anti-Stokes Raman scattering imaging. Opt. Express 2010, 18(12), 13017–28.
- Wang, Z.; Gao, L. et al. Coherent anti-Stokes Raman scattering microscopy imaging with suppression of four-wave mixing in optical fibers. Opt. Express 2011, 19(9), 7960–70.
- Wang, Z.; Liu, Y. et al. Use of multimode optical fibers for fiber-based coherent anti-Stokes Raman scattering microendoscopy imaging. Opt. Lett. 2011, 36(15), 2967–9.
- Balu, M.; Liu, G. et al. Fiber delivered probe for efficient CARS imaging of tissues. Opt. Express 2010, 18(3), 2380–8.
- Legare, F.; Evans, C.L. et al. Towards CARS Endoscopy. Opt. Express 2006, 14(10), 4427–32.
- Saar, B.G.; Johnston, R.S. et al. Coherent Raman scanning fiber endoscopy. Opt. Lett. 2011, 36(13), 2396–8.
Liang Gao, Ph.D., is a former research fellow of the Methodist Hospital Research Institute (TMHRI) (Houston, TX) and is currently Application Scientist at Chroma Technology Corp. (Bellows Falls, VT); e-mail: [email protected]. Zhiyong Wang, Ph.D., is Assistant Member of Department of Systems Medicine and Bioengineering, TMHRI; e-mail: [email protected]. Stephen T.C. Wong, Ph.D., PE, is Chair and Professor, Department of Systems Medicine and Bioengineering; Director, Ting Tsung and Wei Fong Chao Center for Bioinformatics Research and Imaging for Neuroscience (BRAIN); Director, NCI Center for Modeling Cancer Development at TMHRI, and John S. Dunn Distinguished Endowed Chair in Biomedical Engineering, The Methodist Hospital, Weill Cornell Medical College of Cornell University (affiliated with TMHRI), 6670 Bertner St., Houston, TX 77030, U.S.A.; tel.: 713-441-5884, fax: 713-441-8699, e-mail: [email protected]. This research was funded partially by the John S. Dunn Research Foundation and Ting Tsung and Wei Fong Chao Family Foundation (to S.T.C. Wong).