Chlorophenoxy acids are an important class of herbicides that are widely used for weed control in agriculture and forestry. Most of these compounds exhibit a low to moderate order of toxicity in humans at varying degrees and a few are also known to be teratogenic. The two most common acid herbicides of this class, 2,4-D and silvex, are usually found at trace levels in many U.S. groundwaters. Several chlorophenoxy acid herbicides are currently under regulatory control in drinking water by the U.S. Environmental Protection Agency (EPA).
Analytical methods to identify and quantitatively measure these substances in water involve their extractions into a suitable solvent followed by chromatographic analysis. The extraction methods mostly include liquid–liquid extractions (LLE) or solid-phase extractions (SPE). The compounds in the extract are determined by GC using an electron capture detector (ECD), HPLC, GC-MS, or capillary electrophoresis (CE). Derivatization of these acids, usually to their ester derivatives, is often required to improve the chromatographic separation of such herbicides and obtain better sensitivity. Although the GC-ECD and the HPLC methods provide high sensitivity and lower limits of detection, the GC-MS technique is the most authentic confirmatory tool for identification of such compounds. Several derivatization reagents are well-known, and in most cases the acid herbicides are converted into their methyl esters. Such common derivatizing reagents used in environmental trace analysis of acid herbicides are diazomethane1–5 and methanol-containing boron trifluoride. 6–8 Other esterifying reagents that are known to form methyl esters include methanol-H2SO4,9,10 dimethyl sulfate,11 methylchloroformate,12 methyl iodide,11 and benzyltrimethylammonium chloride.13 Esterifying reagents that have been successfully applied to produce other alkyl or aryl esters include 2- chloroethanol-H2SO4,9 pentafluorobenzyl bromide (PFBBr),2,14–16 benzyl bromide,11 and many tetraalkyl ammonium (TAA) salts.17 Esterification of herbicide acids in situ, especially with TAA salts under large-volume on-line/on-column injection conditions, have been reported. The method, however, has been applied selectively to measure a few herbicides only and often requires the presence of sodium hydroxide. It may be noted that diazomethane, the most widely employed derivatizing substance, is toxic, carcinogenic, and explosive. Many other derivatizing agents are toxic as well, and practically all derivatization methods known to measure acid herbicides in environmental waters are laborious, time-consuming, and cumbersome.
In the present investigation, the authors have focused on a simple approach to modify a known method to routinely analyze acid herbicides in environmental waters by GC-MS. Seven acid herbicides were investigated in the study. In this method, the aqueous samples were first acidified and then shaken with methylene chloride in a separatory funnel. The acid herbicides were partitioned from water into the methylene chloride phase and thereby were extracted into this solvent by LLE in one single extraction. Methanol was then added to the methylene chloride extract of acid herbicides along with one drop of 1:1 HCl. The acids were converted into their methyl esters during the evaporation of the solvent extract to a small volume prior to their analysis by GC-MS. This method avoids the use of any costly or hazardous derivatizing agent and seems to be simple and straightforward in approach and practical application.
Experimental
All acid herbicide standards were prepared in acetone. Certified standard solutions at concentrations of 1000 μg/mL were procured from Supelco Inc. (Bellefonte, PA). The standard solutions were further diluted in acetone to give secondary standards. The latter were spiked into 1 L of reagent-grade water to produce appropriate concentrations of herbicides for this investigation. One-liter aliquots of prepared herbicide samples were acidified and then extracted with methylene chloride. The samples were acidified with 5 mL 1:1 HCl prior to their extractions. A single extraction was carried out in a 2-L separatory funnel with 50 mL methylene chloride. In a few experiments, the volume of the solvent was increased to 75 mL. Methylene chloride extracts containing the herbicides in their acid form were passed through a bed of anhydrous sodium sulfate and evaporated to approx. one-half their initial volume on a hotplate. After cooling down to ambient temperature, 5 mL methanol and one drop of 1:1 HCl were added to the extracts to convert the acid herbicides into their methyl esters. The solutions were then swirled for 1 min and further evaporated to a small volume between 1 and 2 mL. The solvent extracts were then passed through a thin bed of anhydrous Na2SO4 to remove any water from the solutions. The final volumes of the solvent extracts were precisely measured.
A 5-μL aliquot of sample extract was injected onto a GC column for analysis. The methyl esters were identified from their mass spectra and retention times. Also, other products formed from any competing side reactions were identified from their mass spectra. The compounds were quantified by analyzing their standard solutions. The GC and MS conditions are outlined below:
- GC column: PTE-5 (Supelco Inc.), 30 m length, 0.25 mm i.d.
- Temperature: oven, 50 °C for 6 min, 8 °C/min to 210 °C, final time, 15 min; injector, 250 °C; detector, 280 °C
- MS conditions: electron impact ionization; electron energy, 70 V; scan mode, 1.57 scans/sec; mass range, 35–550.
Results
Extraction of seven acid herbicides in aqueous matrix by LLE followed by GC-MS determination under scan mode was applied to develop a simple, rapid, and confirmatory method for their analysis. While one of these herbicides, dicamba, was a methoxychlorobenzoic acid, the other six herbicides were the chlorophenoxy derivatives of acetic, propanoic, and butanoic acids. The common names, chemical names, CAS registry numbers, and chemical structures of these compounds are listed in Table 1.
Table 1 - Name and chemical structure of acid herbicides studied

Acidification of samples to a pH below 2 partitioned all acid herbicides into the extraction solvent, methylene chloride. One single extraction from 1 L of aqueous sample taken in a 2-L separatory funnel using a 50-mL aliquot of methylene chloride sufficed for this purpose. However, increasing the volume of the solvent from 50 to 75 mL enhanced the efficiency of extraction by approx. 10%. No further attempt was made in this study to increase the quantity of solvent above 75 mL. Keeping the solvent volume low makes the process cost-effective and shortens the time needed for sample concentration in routine analysis.
This study indicates that the herbicides in the solvent extract may also be determined in their acid form by GC-MS without any esterification. Although esterification did not lower the detection limit of the compounds to any appreciable extent, the ester derivatives enhanced the resolution of compounds on the GC column. In a few experiments, another class of esterifying reagents that includes N,N-dimethylformamide diethylacetal, N,N-dimethylformamide propylacetal, and N,N-dimethylformamide dibutylacetal were employed to esterify the acid herbicides. Although such reagents were able to convert the acid herbicides into their alkyl esters, separation of degraded amide residues from the solvent extracts required additional clean-up steps; therefore, any further investigation on potential applications of these esterifying reagents was not pursued further. In a few experiments, n-butanol was employed for esterification under the same experimental conditions. The objective was to generate butyl derivatives that had larger molecular mass ions than their corresponding methyl derivatives, and thus lower the detection level of such herbicides. However, the authors have found that such esterification reactions with n-butanol did not exclusively produce butyl esters since many side reactions occurred, forming products such as 1,1-dibutoxybutane and 1,1-diisopropoxybutane.
Table 2 - Primary and molecular mass ions and retention times of methyl esters of herbicide acids
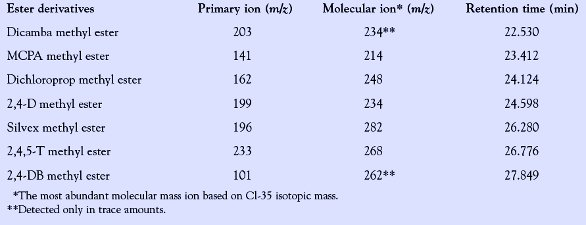
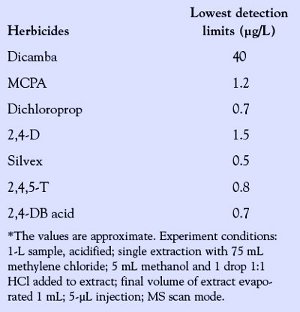
Table 3 - Lowest detection limits of acid herbicides*
The results of this study indicate that esterification of acid herbicides with methanol was complete by the time the solvent extract concentrated down to a small volume, and there was no competing side reaction that produced any undesired product(s) to interfere with chromatographic separation of methyl esters. Such esterification readily occurred in the methylene chloride phase in the presence of one drop of 1:1 HCl under heating during concentration of sample extracts. It may be noted that only a minute quantity of HCl, just one drop, was needed to catalyze the esterification of acid herbicides in the solvent extract with methanol. While no reaction occurred in the absence of HCl, increasing the amount of the latter tended to revert the reaction, reducing the yield of the herbicides as methyl esters and reverting back into their acids.
Among the seven acid herbicides studied, six chlorophenoxy alkanoic acids exhibited a lower (minimum) detection level at concentrations varying from 0.5 to 1.5 μg/L than the methoxychlorobenzoic acid, dicamba, which could only be detected at a much higher concentration of 40–50 μg/L, assuming 5 μL of sample extracts, respectively, were injected onto the GC. The primary and the molecular ions of the methyl esters of acid herbicides and their retention time are presented in Table 2. The minimum detection levels of the seven herbicides tested under the conditions of these experiments are shown in Table 3.