Many samples contain not only neutral hydrophobic analytes, commonly determined using reversed-phase (RP) HPLC, but ionic analytes. Traditional RP-HPLC with a C8 or C18 stationary phase is often unable to simultaneously determine both neutral and charged analytes. Mixed-mode stationary phases, i.e., those possessing reversed-phase and ion-exchange properties,1,2 are ideal for these applications, but many early examples of this phase type suffered from poor column-to-column reproducibility.
The Acclaim® Mixed-Mode WAX-1 (Dionex Products, Sunnyvale, CA) was developed to allow a simultaneous reproducible determination of neutral and anionic compounds in a wide variety of samples. The reproducibility is derived mainly from the unique construction of the stationary phase. Rather than being a mixture of two separate phases (i.e., a reversed-phase and an anion-exchange phase), two separate stationary phase functionalizations, or a phase in which the ion-exchange functionality is embedded and perhaps inaccessible to some molecules, the Acclaim Mixed-Mode WAX-1 column contains a silica-based stationary phase that features a hydrophobic alkyl chain with an ionizable terminus (a weak anion exchanger).1 This construction allows greater control of anion-exchange capacity for better column-to-column reproducibility and facilitates adjustable selectivity for the two retention mechanisms (i.e., RP and anion-exchange modes).
The Acclaim Mixed-Mode WAX-1 has been demonstrated to be an effective solution for a wide range of separation challenges, including samples from the pharmaceutical,3,4 food and beverage,5–7 chemical,8 and other industries.9 The column is especially well suited for separating those compounds that have weak retention on a traditional C18 column and usually require the addition of an ion-pairing reagent to the mobile phase to maintain retention. Selectivity on the Mixed-Mode WAX-1 column is controlled by mobile phase ionic strength, mobile phase organic solvent content, mobile phase pH, and temperature. An increase in ionic strength results in a decrease, an increase, and no change of retention for acidic, basic, and neutral molecules, respectively.9 Hydrophobic retention is markedly affected by organic content of mobile phase, when keeping other conditions constant (e.g., ionic strength, pH, temperature, etc.). Although pH has minimal influence on the retention of neutral molecules, it significantly affects anionic molecules.
In this paper, two sample analyses—determination of iodide in a nutritional supplement (tablet) and determination of bisoprolol and fumarate in a bisoprolol fumarate tablet—are used as examples for demonstrating method development using the Acclaim Mixed-Mode WAX-1 column.
Method development for rapid separation of iodide in a nutritional supplement
Iodide is an essential micronutrient. Supplementing the diet with iodinated salt is most frequently used to add dietary iodide. As well as ion chromatography (IC), which is a commonly used method for determining iodide,10 an HPLC method may be appropriate for some samples. Because of weak iodide retention on the traditional RP C18 column, two published approaches for determining iodide by RP-HPLC show in one paper the addition of an ion-pairing reagent to the mobile phase,11 and in the other paper pre-column derivatization of iodide.12 A simpler approach for the separation of iodide in a complex sample is to use the Mixed-Mode WAX-1 column on which iodide can be retained by anion exchange.
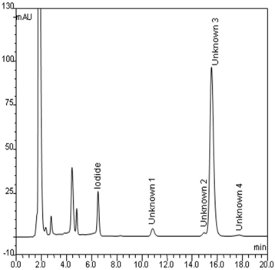
Figure 1 - Chromatogram of the separation of iodide in a nutritional supplement sample (tablet) spiked with iodide standard (10 μg/L). Column, Acclaim Mixed-Mode WAX-1, 3.0 × 150 mm, 3 μm; eluent, 100 mM KH2PO4 (pH 4.5, without adjustment)–acetonitrile (1:1, v/v); column temperature, 30 ºC; flow rate, 0.5 mL/min; injection volume, 5 μL; UV detection, 220 nm.
A widely used initial mobile phase for method development for the Mixed-Mode WAX-1 column is 100 mM of potassium phosphate (pH 4.5)–acetonitrile (1: 1, v/v). Using this mobile phase, the authors achieved good separation of iodide in a nutritional supplement (tablet) sample that was spiked with an iodide standard (Figure 1), though the analysis time was long due to unknown molecules in the supplement. If it is assumed that the unknowns are neutral molecules, their retention can be decreased and the method can be accelerated by increasing the organic solvent content of the mobile phase. However, since phosphate readily precipitates at high organic solvent content, the authors initially investigated changing the buffer concentration.
Decreasing the potassium phosphate concentration to 50 mM (pH 4.5, adjusted with K2HPO4) caused the retention of iodide and the unknown molecules to increase, e.g., the elution of unknown peak 3 moved to 40 min, demonstrating that at pH 4.5 they are negatively charged. Therefore, changes in column selectivity due to pH value and phosphate buffer concentration of the mobile phase were investigated.
Experiments on the effect of buffer pH value showed that when increasing the buffer pH value, the peaks for iodide and the unknown molecules all eluted earlier, and unknown peak 3 eluted much earlier, demonstrating that a higher pH value of buffer was beneficial for a faster analysis. This is due to the potassium phosphate eluent having a higher elution power at higher pH. Therefore, a buffer pH value of 6.3 was selected for the separation.
Increasing the pH 6.3 phosphate buffer concentration caused a decrease in the retention of the unknown molecules and iodide. The unknown molecules lost more retention than iodide, demonstrating the unknown molecules were highly ionic acidic molecules and likely multivalent. Adjusting the proportion of acetonitrile caused a significant change in iodide retention, while the retention of the unknown molecules was nearly unchanged. At this pH, the separation mode of iodide appears to be mainly RP, and that of the unknown anionic molecules mainly anion exchange, suggesting that the unknowns are hydrophilic anionic molecules.

Figure 2 - Chromatogram of an improved separation of iodide in a nutritional supplement sample (tablet) spiked with iodide standard (10 μg/L). Column, Acclaim Mixed-Mode WAX-1, 3.0 × 150 mm, 3 μm; eluent, 70 mM KH2PO4 (pH 6.3, adjusted with K2HPO4)–acetonitrile (3:1, v/v); column temperature, 30 ºC; flow rate, 0.5 mL/min; injection volume, 5 μL; UV detection, 220 nm.
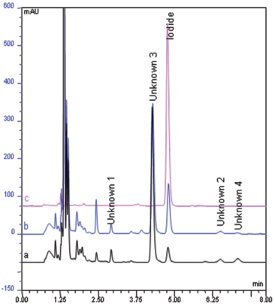
Figure 3 - Overlay of chromatograms: a) A nutritional supplement sample (tablet), b) same sample spiked with iodide standard (10 μg/L), and c) iodide standard (35 μg/L). Column, Acclaim Mixed-Mode WAX-1, 3.0 × 150 mm, 3 μm; mobile phase, 70 mM KH2PO4 (pH 6.3, without adjustment)–acetonitrile (72:28, v/v); column temperature, 40 ºC; flow rate, 0.6 mL/min; injection volume, 10 μL; UV detection, 220 nm.
Based on the above investigations, the authors were able to reduce the separation time from 20 to 14 min (Figure 2). To further decrease the separation time, the flow rate and column temperature were increased. The higher column temperature served to increase the separation speed and decrease the column backpressure caused by the accelerated flow rate. The proportion of phosphate buffer and acetonitrile was adjusted slightly to improve resolution. As shown in Figure 3, the separation was completed within 8 min with good peak resolution and symmetry.
In summary, the authors started with one of the suggested mobile phase conditions, altered the buffer concentration to determine which peaks were being retained by anion exchange, and then investigated the effect of pH. Having arrived at a pH that would promote less retention, they varied the buffer concentration and organic acid content of the mobile phase to evaluate their effect on retention and resolution, and then accelerated the separation by increasing flow rate and temperature.