From the dramatic increase in new fluorophores to the proliferation of techniques like total internal reflection fluorescence (TIRF), photoactivated localization microscopy (PALM), and stochastic optical reconstruction microscopy (STORM), all the signs are clear: Live cell imaging is driving the next generation in biological imaging, and fluorescence is leading the parade. Microscope illumination is the first step in any live cell experiment, and long-time industry innovator Lumen Dynamics Group (LDGI, Mississauga, Canada, previously EXFO LSID) has the next generation: the X-Cite® XLED1.
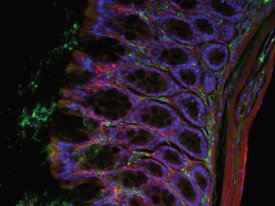
Figure 1 - The new biology requires 2, 3, 4 or more wavelengths. (Image courtesy of Dr. Kavita Asawani, LDGI.) Gut, 20×, Hoechst, Alexa 488 (tubulin), and Cy3 (laminin).
What do research biologists need?
Research biologists have often led the microscopy community in the evolution of new technologies. Key examples include phase contrast, Hoffman Modulation Contrast, differential interference contrast (DIC), and fluorescence itself. Illumination is no different. New biological protocols dictate next-generation parameters such as increased stability and uniformity; longer “lamp” life; less heat to the sample; improved signal-to-noise ratio, and the ability for fast switching between 2, 3, or 4 wavelengths (Figure 1). It would also be nice if there was no wait for the lamp to warm up and stabilize and if the field of view was flat.
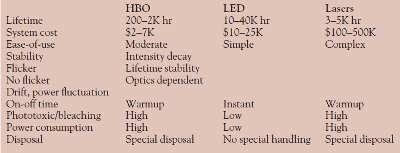
Table 1 - Comparison of HBO, LED, and laser illuminators
The LED solution
As seen in Table 1, light-emitting diode (LED) illuminators answer all of these live cell needs. They enjoy extremely long lifetimes, are economical, and require no special handling or disposal. Running at much lower temperatures than lasers or arc sources, they significantly reduce phototoxicity and bleaching, while their narrow bandwidths optimize excitation.
LEDs taken to the next level
LDGI developed the X-Cite XLED1 to surpass existing LED performance. Working in close collaboration with one of the major microscope manufacturers, the company listened carefully to biologists’ needs. First, a system was engineered to accommodate up to four high-power LEDs to match the wavelengths of the most common fluorophores: DAPI (385 nm), GFP (460 nm), Cy3 (525 nm), and an optional module for Cy5 (635 nm). In actuality, as demonstrated in Table 2, these options cover a broad gamut of dyes, proteins, and probes.
Table 2 LED excitation wavelengths for key dyes, probes, and proteins

Switching between wavelengths is instantaneous, and the system can be programmed to mix any combination of LEDs. The absence of moving parts like shutters and filter wheels eliminates the chatter from vibration, which can be disruptive to live cell imaging. Of equal importance, each LED can be switched off instantaneously when not in use, minimizing photobleaching when the cell is not being imaged.
For reproducibility, intensities can be set on the 7-in. touchscreen display (Figure 2) using arrows, sliders, or specific numerical input for values from 5% to 100%, in 0.1% increments. Figure 2a shows the intensity display, and Figure 2b the system status.
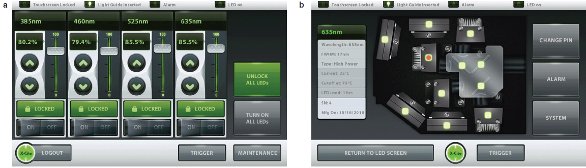
Figure 2 - The X-Cite XLED1 features a 7-in. touchscreen display and intuitive user interface.
Flexibility for multiuser facilities
Flexibility, reliability, and reproducibility are key for any multiuser facility. Because X-Cite XLED1’s liquid light guide (LLG) and connection adaptor fit most existing laboratory and research microscope stands, it can be used for regular fluorescence microscopy as well as conventional and spinning disk confocal, multiphoton, structured illumination, and super-resolution systems. The LLG also ensures that the field will be evenly illuminated from edge-to-edge, which is critical for quantitation and when tiling multiple images to form a larger image.
Individual LED modules are so quick and easy to exchange that even a novice can do it. While X-Cite XLED1 is launching with four modules, additional modules will be available in the near future.
To avoid user error, the LED uses intelligent software and feedback to recognize immediately which module is in which location. Utilizing the user interface, each microscopist can rename wavelengths to fit a fluorophore of choice and with password protection create a customized illumination protocol to fit his or her experiment. The system will remember and reproduce all of the settings.
The light budget
Despite their wattage ratings, not all LEDs are the same. Understandably, the optics in each system affect the actual illumination that falls on the sample. This limitation is especially critical for the very important midspectrum green LEDs, which have not quite caught up to their counterparts in output intensity.
While the best way to judge is to use a focal plane power meter,1 the paddle-style power meter that comes with many lasers is a reasonable alternative. The expansion of LED technology has generated a new term: “$/ mw” or the cost of the system per milliwatt of power falling on the sample. Overall intensity has been a pivotal factor driving the switch from conventional arc lamps to LED illuminators, but higher power is especially important for spinning disk systems and photolysis. Depending on the wavelength used, the X-Cite XLED1 typically produces on the order of 2–5 times greater intensity at the sample than other LED systems. Prof. Chris Yip (University of Toronto, Canada), who conducts both fluorescence and uncaging experiments, reported that it is much easier to use than laser-based systems.
The living cell as a laboratory
Today, the cell is seen as a laboratory. Gone are the days when scientists were only interested in imaging. Using techniques like uncaging, fluorescence resonance energy transfer (FRET), fluorescence recovery after photobleaching (FRAP), and the new photoactivated (PA) modalities,2 they want to study processes, adjacencies, and interactions. To meet those needs, X-Cite XLED1 uses a programmable pulse controller with single-pulse or modulated pulse train triggering options. Pulses can be as short as 10 μsec or as long as 18 hr. A global trigger input controls all wavelengths simultaneously and can be activated internally, or, using the TTL protocol, through external pulse generators. Beyond the illumination system, carefully choosing fluorophores with well-separated emission curves and using a multiwavelength or notch filter on the emission side also maximize speed.
In addition to receiving signals, the X-Cite XLED’s sync-out can send signals to other peripherals such as a camera or microscope stage. Externally, the LED can be controlled through many widely used software packages. Alternatively, the more independent user can program the system using a software development kit (SDK).
Why switch to an LED system?
Despite being innovators, research biologists are also conservative. As a group, we have been using mercury arc technology for well over half a century. We tend to hold onto our equipment, often for decades. Also, LED systems are not inexpensive, so why switch? The answer is as simple and as complex as the information produced by one image. The light is more controlled and more consistent. LED systems produce flat, evenly illuminated fields, without flicker and with excellent signal-to-noise ratios that allow the biology to shine through against a rich black background. The results are more readily quantified and consistent over time and from laboratory to laboratory. They produce light that is less toxic to samples and can be programmed and managed to a microsecond, without the disruptive chatter and vibration of shutters or filter wheels.
Finally, just as fluorescent probes have blossomed to allow biologists to research more and more advanced topics, LEDs are blooming in a rainbow of colors to keep pace. As epitomized by the X-Cite XLED1, the technology has matured rapidly over the past five years, and while arc sources and lasers still have very important roles, LEDs are emerging as the preeminent choice for research biology.
References
- Foster, B. Fluorescence illumination: new metrics for reproducibility and quantitation. BioPhotonics Jul/Aug 2011, 34–7.
- Application note. Calibrating photouncaging in an ATR-FTIR spectrometer using the X-Cite® XLED1, the X-Cite® XR2100 power meter, and the XP750 objective plane power sensor. Download from www.ldgi-xcite.com.
Ms. Foster is President and Chief Strategic Officer, The Microscopy & Imaging Place, Inc., McKinney, TX, U.S.A., and Consulting Editor, American Laboratory/Labcompare; tel.: 972-924-5310; e-mail: [email protected].