Discussions on the use of the Tag-lite® platform (Cisbio Bioassays , Codolet, France) for G protein-coupled receptor (GPCR) applications have revolved solely around affinity or potency and the calculation of the Kd output, greatly undermining the kinetic aspect of the binding process. Kd and Ki refer to the equilibrium constant, and thus reflect an equilibrium state. In contrast, koff and kon rates allow the prediction not only of the equilibrium state of a drug, but also of how fast the drug–receptor system responds to changes in the concentration of the drug or to another competitor.
This article describes use of the Tag-lite dopamine D2 ligand binding kit to calculate the association and dissociation rates of spiperone-d2. The flexibility and throughput of the Taglite platform enable fast and easy calculation of the koff and kon rates for a given receptor.

Figure 1 - Spiperone-d2 association binding experiment. Frozen labeled cells #C1TT1D2 (HEK 293 cells expressing D2 receptor fused with SNAP-Tag® [New England Biolabs, Inc., Ipswich, MA], labeled with europium cryptate) were incubated at room temperature at different times with increasing concentration of spiperone-d2 ligand. Specific binding was measured for each ligand concentration.
Materials and methods
Saturation binding and association rate experiments
Labeled spiperone (spiperone-d2, Cisbio #L002RED) was reconstituted as described in Cisbio’s recommendations in Tag-lite labeling medium (#LABMED) and was diluted twofold from a 100 nM solution to a 1.5 nM solution. Frozen labeled cells (Tag-lite dopamine D2-1 million labeled cells, #C1TT1D2) were reconstituted following Cisbio’s recommendations and diluted in Tag-lite labeling medium to a 200 cells/μL concentration. Affinities of the fluorescent ligand for the receptor were determined by incubating the cells at room temperature (RT) with increasing concentrations of labeled ligand (LL). For each fluorescent ligand concentration, the nonspecific binding was determined by adding a large excess of unlabeled spiperone. Reagents were dispensed following Cisbio’s ligand binding protocol in 384-well format (white plates). Assay plates were incubated at RT and read at different time points thereafter on an HTRF® certified reader (Cisbio). Plots were fitted in Prism4 (GraphPad Software Inc., La Jolla, CA) with a one-phase exponential association curve.
Table 1 - Association pharmacokinetic values of spiperone-d2

Dissociation rate experiment
Dissociation was initiated after the system reached equilibrium (1 hr after ligand addition) by adding 3 μL of a 100 μM bromocriptine solution in 3% dimethyl sulfoxide (DMSO). The final concentration of bromocriptine was 13 μM for a final 0.02% DMSO concentration, well in excess of the labeled ligand concentration. Measurements of the plate, incubated at RT, were distributed over a lengthy period of time in order to capture dissociation. In each case, the effect of dilution over time was plotted to obtain the time for a 50% decline in binding (t1/2). The plate was reread until dissociation was complete. Plots were fitted in Prism4 with a one-phase exponential decay curve.
Results
Kd determination by saturation binding experiment
The HTRF ratio was measured for each labeled ligand concentration. The specific signal was obtained by subtracting the nonspecific signal from the total signal. The Kd value for spiperone-d2 was calculated after the system reached equilibrium, i.e., after 60 min of incubation, and was found to be 8 nM.
kon and koff determination by association experiments
Association binding experiments were used to determine the association rate constant or kon by addition of spiperone-d2. The specific binding was measured at various times (Figure 1), and the calculated parameters are presented in Table 1.

Figure 2 - Dopamine D2 receptor saturation binding experiment. Frozen labeled cells #C1TT1D2 (HEK 293 cells expressing D2 receptor fused with SNAP-Tag, labeled with europium cryptate) were incubated for 1 hr at room temperature with increasing concentration of spiperone-d2 ligand. Specific binding was measured for each ligand concentration.

Figure 3 - kon and koff were determined. kobs was measured at different ligand concentrations and represented as a function of ligand concentration. Pharmacokinetics parameters were determined. kon was the slope and koff was the value of kobs extrapolated at the origin.
Binding increases over time, then levels off after 60 min of incubation (Figure 2). For each labeled ligand concentration tested, a kobs value was derived, and was later transformed into a kon rate based on the ligand concentration and the koff rate.
The observed association rate constant, kobs, is also defined by the following equation:
As the concentration of labeled ligand increases, the observed rate constant or kobs should increase linearly (as opposed to the fixed constant koff). Figure 3 represents kobs as a function of the labeled ligand concentration for the dopamine D2 receptor and spiperone-d2. kon and koff were determined from this figure, and the Kd were then calculated as the koff/kon ratio.
Dissociation experiment to calculate koff
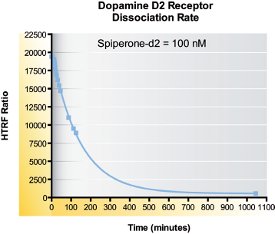
Figure 4 - Spiperone-d2 100 nM was incubated for 1 hr to reach equilibrium. Dissociation was initiated by an excess of unlabeled bromocriptine, and specific binding was measured at different times.
A dissociation binding experiment measures the “off rate” (koff) for ligand dissociating from the receptor. Initially, ligand and receptor were allowed to bind until equilibrium was reached. At that point, dissociation was initiated by massive introduction of unlabeled bromocriptine, a full agonist of dopamine D2 receptor (Figure 4).
Binding decreased over time until it leveled off after overnight incubation. koff values were derived and were later used to calculate the kon rate. From the fit, Prism4 also calculates a dissociation half-time (t1/2) in minutes–1 (Table 2). The dissociation half-time represents the time needed for half the ligands to dissociate from the receptor to which they were initially bound. Table 2 summarizes the values obtained during the dissociation experiment for spiperone-d2. koff was obtained directly from the dissociation experiment, while kon and Kd were calculated.
Table 2 - Dissociation pharmacokinetic values of spiperone-d2
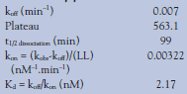
Table 3 - Comparison of the Kd determination by saturation experiment or pharmacokinetic experiments; Kd calculated after the pharmacokinetic experiments is the ratio koff/kon

Discussion
The dissociation half-life, using the dilution method, was 99 min; the koff rate 0.00699 min–1. These results correlate well with the results for unconjugated spiperone as measured using radioactivity by Kapur and Seeman.1
Reported spiperone t1/2 was 200 min (by excess of raclopride, an antagonist ligand for the dopamine D2 receptor) and koff rate was 0.003 min–1. These results matched the results obtained with the dilution method. The Kd calculated from the saturation binding experiment (Figure 2), the association experiment (Figure 1), and dissociation experiment (Table 2) were similar (Table 3). This confirms that the dopamine D2 binding assays developed with the Tag-lite reagents follow the law of mass action.
Conclusion
Tag-lite was utilized to directly measure the kinetics of spiperone-d2 binding on the cell surface-expressed dopamine D2 receptor. The platform enables generation of the dissociation and association rates for a given labeled molecule in a plate-based homogeneous format. The pharmacokinetic values obtained were in agreement with the published literature and matched the equilibrium constant obtained from saturation binding experiments.
Reference
- Kapur, S.; Seeman, P. Antipsychotic agents differ in how fast they come off the dopamine D2 receptors. Implications for atypical antipsychotic action. J. Psych. Neurosci.2000, 25(2), 161–6.
Mr. Pierre is a Scientific Consultant, Cisbio US, Inc., 135 South Rd., Bedford, MA 01730, U.S.A.; tel.: 888-963-4567; e-mail: [email protected].