Trace-level determination of toxic compounds from complex matrices often requires a sample cleanup step such as liquid–liquid extraction (LLE) or solid-phase extraction (SPE) prior to analysis in order to clean and preconcentrate the sample. While historically LLE has been the preferred technique for cleanup, it is time consuming, and the solvents employed have frequently involved environmental and health hazards. SPE using nonpolar, polar, ionic, or mixed-mode (e.g., nonpolar and ion exchange groups) sorbents is more rapid, simple, and economical than traditional LLE. Even so, the hydrophobic, ionic, and polar sorbents most widely used typically do not provide high enough selectivity, resulting in tedious sample preparation methods and low throughput.
SPE utilizing molecularly imprinted polymer (MIP) sorbents has been growing rapidly in the past few years, and there are now several publications in the literature that cover a large number of different types of toxic compounds, such as banned food contaminants (beta-agonists,1 chloramphenicol,2,3 and mycotoxins4), environmental contaminants (triazines5 and beta blockers6), and compounds in tobacco smoke (nicotine,7–9 cotinine,10 and the tobacco-specific nitrosamine (TSNA) 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL).11 This article shows comparative studies on the extraction of NNAL, the human metabolite of the TSNA 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), and the fourdifferent TSNAs (N′-nitrosonornicotine [NNN], NNK, N′-nitrosoanabasine [NAB], and N′-nitrosoanatabine [NAT]) from urine samples using SupelMIP cartridges (MIP Technologies AB, Lund, Sweden) versus conventional mixed-mode cation-exchange polymer SPE cartridges.
Technical description of MIPs
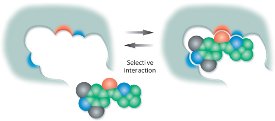
Figure 1 - Schematic of the imprinting site.
Molecularly imprinted polymers are a class of highly cross-linked polymer-based molecular recognition elements engineered to bind one target compound or a class of structurally related compounds with high selectivity. Selectivity is introduced during MIP synthesis in which a template molecule, designed to mimic the analyte, guides the formation of specific cavities or imprints that are sterically and chemically complementary to the target analyte(s). After complete polymerization, sophisticated wash procedures are used to remove the template from the polymer, leaving the imprints or binding sites accessible to bind the analyte(s) of interest (see Figure 1).
The design of the imprinting site is carried out either by molecular modeling, experimental design (DOE), or empirical screening methods, and leads to cavities with multiple noncovalent interaction points based on ion pairing, hydrogen bonding, and hydrophobic interactions between the analyte and the MIP polymer material, resulting in strong and specific analyte retention. This selectivity is exploited during the extraction procedure, where harsh washing conditions can be employed during extraction for the removal of interferences. This, in turn, leads to cleaner extracts, lower detection limits, and a more efficient sample cleanup process.
Extraction of tobacco-specific nitrosamines from urine samples
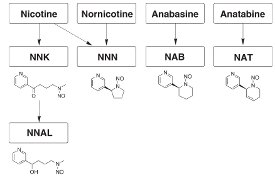
Figure 2 - Metabolism of tobacco smoke.
The monitoring of humans for exposure to tobacco smoke (active or passive) is an important clinical test. Nicotine is metabolized in the tobacco curing process to the TSNAs NNK and NNN; NNK is further metabolized in the body to NNAL (see Figure 2). These compounds, together with NAB and NAT, deriving from other alkaloids during tobacco curing, are important in testing exposure of an individual to tobacco smoke. MIPs are a powerful tool in the cleanup of urine samples in order to achieve the detection limits needed to test for these biomarkers. By careful design of the imprinting site, the binding cavities of the SupelMIP may be engineered to offer compound-specific or class-specific sample cleanup. The compound-selective SupelMIP SPE-NNAL and the class-selective SupelMIP SPE-TSNA are discussed in more detail below.
Compound-selective SupelMIP SPE-NNAL
The analysis of NNAL is challenging due to the detection limits required, especially in the monitoring of nonsmoker exposure to second-hand tobacco smoke. In the case of nonsmokers, detection limits below 10 ng/L (<10 ppt) for NNAL are required. Such low detection limits are not attainable using conventional phase SPE cleanup, even with MS-MS detection. Some methods have been reported that approach the detection limits needed, but these involve laborious sample cleanup, often taking up to three days, and necessitate the use of expensive specialized detectors.
The SupelMIP SPE-NNAL is designed to offer compound-specific binding sites for the NNAL molecule.11 This specificity allows for significantly better cleanup than conventional SPE phases. Conventional SPE phases utilize nonselective interactions through hydrophobic and ionic groups as the basis for the extraction of NNAL. Using such low-specificity interactions, sufficiently clean extracts that permit the required detection limits or accuracy are not obtained. The SupelMIP SPE-NNAL, on the other hand, is designed to allow specific interactions with the functionality and stereochemistry of the NNAL molecule, and provides a multiple interaction binding site that enables strong differentiation between NNAL and other related interferents in the matrix. Stronger wash solutions can therefore be used, leading to much cleaner extracts, which in turn allows for better detection limits.
Cleanness of extract
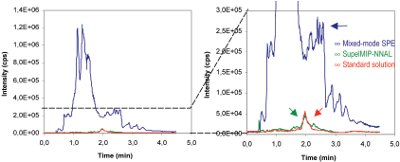
Figure 3 - Comparison of extracts from SupelMIP NNAL (green line) and mixed-mode polymeric SPE (blue line). A Q1 MS scan for 50 ppt NNAL at m/z 210 amu was analyzed. The extracts from both cleanup methods are compared to a standard prepared in mobile phase (red line). NNAL retention time was 2.0 min. Using the SupelMIP procedure, clean extracts were obtained containing very low amounts of interfering contaminants.
The extracted samples were tested for cleanness by checking a Q1 MS scan at 210 amu (NNAL molecular weight). This scan reveals any interferences that are ionized and pass into the MS detector in addition to NNAL.
Figure 3 illustrates the Q1 scan of a triple quadruple MS-MS instrument when the mass ion for NNAL (m/z 210 amu) is sampled. The cleanness of the extract from a urine sample using the SupelMIP NNAL (green line) is significantly better than a conventional mixed-mode polymeric SPE phase (blue line). This allows for better accuracy and fewer matrix effects, and achieves the detection limits required to monitor nonsmoker exposure to tobacco smoke (<10 pg/mL).
Recoveries
Table 1 - Absolute recovery of SupelMIP SPE-NNAL vs mixed-mode polymeric SPE (without internal standard adjustment)

The absolute recoveries achieved from both methods were calculated and are shown in Table 1. Recoveries using the SupelMIP are much higher than the mixed-mode polymeric SPE. Internal standards were not used in the calculation in order to provide an indication of the absolute recovery of each method. However, it is recommended that relative recovery be quantitated against NNAL-d3 internal standards that are spiked into urine samples prior to SupelMIP extraction. Under such conditions, analysts can readily achieve relative recovery values >90% and limits of quantitation (LOQ) of 5 pg/mL. For the mixed-mode polymeric SPE, the LOQ value was 20 pg/mL.
Class-selective SupelMIP SPE-TSNAs
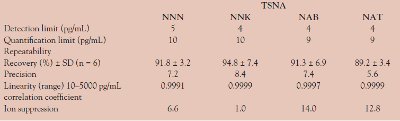
Table 2 - Summary of performance for analytical method for the determination of TSNAs in urine using SupelMIP SPE–TSNA
The four TSNAs—NNK, NNN, NAB, and NAT—are important elements in human exposure to tobacco smoke.12 TSNAs are known carcinogens and, since there are several related TSNAs, formed from precursors present in tobacco, an analytical method that is class selective for TSNAs is more relevant. The SupelMIP TSNA was developed as such a class-selective MIP. The binding sites were designed to provide specific recognition of the common substructure of the TSNAs. Once the MIPs were obtained, the SPE cleanup method using SupelMIP TSNA was critically optimized in order to obtain the cleanest TSNA extract from urine.
A summary of the performance of the analytical procedure13 is given in Table 2. Detection limits of 5 pg/mL are obtainable for all TSNAs from urine samples. Recovery values for each TSNA are better than 90%, and ion suppression due to matrix effects is low. Such performance permits accurate determination with the required precision of measurement of TSNAs in urine using a simple, one-step SPE cleanup step.
Conclusion
A novel method for the extraction of NNAL and TSNAs NNN, NNK, NAT, and NAB from urine using SupelMIP SPE-NNAL and SupelMIP SPETSNA was described. A comparison was made with a mixed-mode cation exchange polymer phase. Due to the selective interactions on the SupelMIP phase, stronger binding occurs between the analytes and the MIP sorbent than are possible in the mixed-mode phase. This makes it feasible to use stronger wash solutions, resulting in more effective removal of interfering compounds, lower detection levels, minimized ion suppression, less frequent cleaning of the MS source, and a more efficient cleanup procedure.
References
- Van Hoof, N.; Courtheyn, D.; Antignac, J.-P.; Van de Wiele, M.; Poelmans, S.; Noppe, H.; De Brabander, H. Multi-residue liquid chromatography/tandem mass spectrometric analysis of beta-agonists in urine using molecular imprinted polymers. Rapid Comm.Mass Spec. 2005, 19, 2801–8.
- Mohamed, R.; Richoz-Payot, J.; Gremaud, E.; Mottier, P.; Tabet, J.C.; Guy, P.A. Advantages of molecularly imprinted polymers LC-ESIMS/MS for selective extraction/quantification of chloramphenicol in milk. Comparison to a classical sample preparation. Poster presented at ASMS 2007, June 3–7, 2007, Indianapolis, IN.
- Shimelis, O.; Trinh, A.; Brandes, H. The selective extraction of chloramphenicol using molecular imprinted polymers. Supelco Reporter2007, 25.1, 11–13. www.sigmaaldrich.com/supelmip.
- Appel, M.; Kendra, D.F.; Kim, E.K.; Maragos, C.M. Synthesis and evaluation of molecularly imprinted polymers as sorbents of moniliformin. Food Additives and Contaminants2007, 24(1), 43–52.
- Matsui, J.; Okada, M.; Tsuruoka, M.; Takeuchi, T. Solid-phase extraction of a triazine herbicide using a moleculary imprinted synthetic receptor. Anal. Comm. 1997, 34, 85-7.
- Pizzolato, T.M.; Gros, M.; Petrovic, M.; López de Alda, M.J.; Barceló, D. Trace level determination of beta-blockers in natural waters by highly selective molecular imprinted polymers (MIP) followed by liquid chromatography-tandem mass spectrometry quadropole-linear ion trap mass spectrometry. 3rd SWIFT-WFD (Water Framework Directive) Workshop2006, May 15–16, 2006, Barcelona, Spain.
- Zander, Å.; Findlay, P.; Renner, T.; Sellergren, B. Analysis of nicotine and its oxidation products in nicotine chewing gum by a molecularly imprinted solidphase extraction. Anal. Chem. 1998, 70, 3304–14.
- Mullet, W.M.; Lai, E.P.C.; Sellergren, B. Determination of nicotine in tobacco by molecularly imprinted solid phase extraction with differential pulsed elution. Anal. Commun. 1999, 36, 217–20.
- Yang, J.; Hu, Y.; Cai, J.B.; Zhu, X.L.; Su, Q.D.; Hu, Y.Q.; Liang, F.X. Selective hair analysis of nicotine by molecular imprinted solid-phase extraction: an application for evaluating tobacco smoke exposure. Food Chem. Toxicol.2007, 45, 896–903.
- Yang, J.; Hu, Y.; Cai, J.-B.; Zhu, X.-L.; Su, Q.-D. A new molecularly imprinted polymer for selective extraction of cotinine from urine samples by solidphase extraction. Anal. Bioanal. Chem. 2006, 384, 761–8.
- Xia, Y.; McGuffey, J.E.; Bhattacharyya, S.; Sellergren, B.; Yilmaz, E.; Wang, L.; Bernert, J.T. Analysis of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in urine by extraction on a molecularly imprinted polymer column and liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Anal. Chem.2005, 77, 7639–45.
- Stepanov, I.; Hecht, S.S. Tobacco-specific nitrosamines and their pyridine-N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol. Biomarkers Prev.2005, 14(4), 885–91.
- SupelMIP SPE- TSNAs Instruction Sheet T706031,www.sigmaaldrich.com/supelmip.
Dr. Widstrand is VP, Sales & Marketing; Mr. Boyd is Manager, Analytical Chemistry; Dr. Billing is Senior Scientist; and Dr. Rees is CEO, MIP Technologies AB, Scheelevägen 22, 220 07 Lund, Sweden; tel.: +46 46 163900; fax: +46 46 163901; e-mail: [email protected].