Uniquely quantifying varying levels of acidity or basicity in polyol samples has traditionally required two different methods utilizing less than desirable titrants with less than optimal results. These parameters are extremely important production determinations, since the quality of yield is dependent on the small variances in these results.
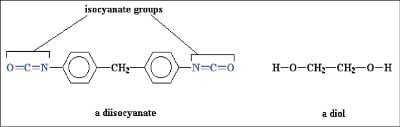
Figure 1 - A diisocyanate and a diol. The diisocyanate has two cyanate groups (shown in blue), and the diol has two alcohol groups.
Polyols are chemical compounds, usually linear, containing multiple hydroxyl groups. When these hydroxyl groups are made readily available for chemical reaction, they can formulate an array of different products ranging from food additives to fabrics to plastics. Many of the most common products associated with this type of reaction are polyurethanes, which are formulated by reacting a diisocyanate and a polyol in the presence of a catalyst under tight acidity control (see Figure 1) . Monitoring the levels of acidity and basicity of the raw polyol products is important to catalyze the polyurethane reaction, and if not correctly controlled, can lead to undesired side reactions.
The traditional method for determining acidity/basicity in a nonaqueous matrix is to utilize alcoholic KOH and HCl in two distinctive titration methods. One of the limitations in this chemistry is the inability to quantitatively differentiate between acidity and basicity in a single analysis. Furthermore, KOH readily reacts with atmospheric carbon dioxide to form carbonates and bicarbonates, rapidly distorting its integrity. These two limitations were direct factors in METTLER TOLEDO’s (Columbus, OH) teaming up with Dr. Ross Koile to perfect an analytical test method that not only differentiates acidity from basicity in a single test method, but also can yield quantitative levels of both while at the same time eliminating the undesirable KOH titrant. The importance of this single test method is amplified by the fact that the desired range of acidity/basicity of the raw polyol is usually exactly neutral or marginally acidic.
The basics of the chemistry are quite simple. A known amount of an atmospherically stable bicarbonate solution is dispensed and titrated with p-toluenesulfonic acid. If the polyol samples are acidic, they will react immediately with the bicarbonate dispensed as part of the back titration. This results in the amount of titrant needed to reach the equivalence point being less than the blank. If the polyol sample is basic, it will not react with the bicarbonate dispensed, resulting in an excess of titrant needed to neutralize the sample.
A key to the analysis is to define neutrality. In an aqueous system, an electrode filled with KCl as the reference solution should have an isopotential point very close to 0 mV. However, as nonaqueous reference solutions are substituted for KCl, the isopotential changes. This phenomenon requires us to define neutrality in nonabsolute mV terms. For this analysis, the bicarbonate/carbonic acid couple was chosen to define neutrality. It turns out that this system can be used in a nonaqueous environment, particularly if the solvent is polar.
Next, a suitable solvent must be chosen in which the sample is soluble and optimal for electrode performance. The sample in this case is a polyether glycol that is soluble in most alcohols. The larger the alkyl chain of the alcohol (to a point), the better the solubility of the polyol. The solubility of this material in methanol is somewhat limited, but ethanol and the propanols are quite good. The cost of obtaining anhydrous ethanol can be a problem, and while both propanol and isopropanol are very good as solvents, their viscosity slows electrode response. The optimal solution is to mix equal volumes of methanol and isopropanol. The isopropanol allows for excellent sample solubility, and the methanol reduces the overall solution viscosity so that the electrode will respond in a reasonable time frame. Some longer-chain polyols will necessitate an extra solubility push for full dissolution of the sample into solution. In these cases, tetrahydrofuran (THF) stabilized with butylated hydroxytoluene (BHT) is an excellent choice. A very easy way to tell if tetrohydrofuran is required is to look at the titration curve in its absence. If the sample is not fully dissolved, the curve will be very flat and demonstrate a very shallow inflection (Figure 2). An immediate addition of THF will solve this solubility issue (Figure 3).
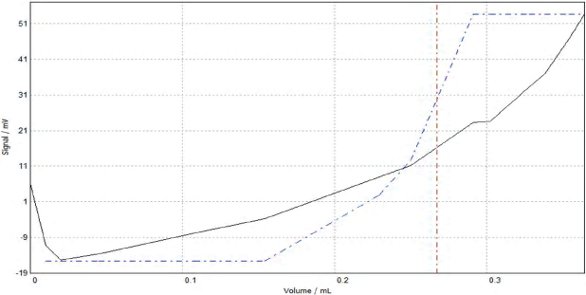
Figure 2 - Sample needing THF.

Figure 3 - Typical sample curve.
Good solution conductivity is another key to this analysis when making potentiometric. If this were an aqueous solution, we would just add a salt to the mixture to increase conductivity. Most ionic salts are not soluble in alcoholic solutions; however, lithium chloride is very soluble in the lower alcohols and solves the problem. There are other salts that can be used (such as quaternary ammonium salts), but we need something that will not influence the acid/base balance of this solvent mixture.
The choice of titrant needs to take into account that the matrix of the sample is nonaqueous in nature and demonstrate a very shallow inflection. An immediate addition of THF will solve this solubility issue. Good solution conductivity is another key to this analysis when making potentiometric measurements. If this were an aqueous solution, we would just add a salt to the mixture to increase conductivity. Most ionic salts are not soluble in alcoholic solutions; however, lithium chloride is very soluble in the lower alcohols and solves the problem. There are other salts that can be used (such as quaternary ammonium salts), but we need something that will not influence the acid/base balance of this solvent mixture. The choice of titrant needs to take into account that the matrix of the sample is nonaqueous in nature and thus the matrix of the titrant should also be nonaqueous. The introduction of an aqueous titrant could make the water content of the final sample/solvent/titrant solution so high that the “leveling” effect of water could become an issue, which suppresses the sharpness of the inflection. The question of whether an acid or base should be used is an easy one, considering that bases need protection from atmospheric carbon dioxide and acids do not. The acid needs to be strong and soluble in polar organic solvents. p-Toluenesulfonic acid works well for this application. As mentioned earlier, the matrix of the titrant should be nonaqueous to limit water content. Alcohols would be the first logical choice; however, p-toluenesulfonic acid reacts with alcohols to form esters, which drastically affects the stability of the solvent. Thus, an aprotic solvent would be a better choice since it prevents the esterification reaction, leading to greater solution stability. Acetonitrile fits this requirement very well and is optimal considering the acid’s solubility as well as its availability in an anhydrous state. Additionally, the dynamic method of standardization is recommended.
The titration solvent is equal part mixture of methanol and isopropanol, with the addition of THF in cases of long-chain polyols. The solution is enhanced with a small amount of 0.25 g/L LiCl and a very small amount of aqueous 0.2 M NaHCO3. The titrant is 0.005N p-toluenesulfonic acid in acetonitrile. The electrode needs to have a fast flow rate filled with ethanolic LiCl. Analytical results can be either positive or negative. Positive results indicate a basic sample, and negative indicate acidic. The sample should be taken and stored in polyethylene bottles because this procedure is sensitive enough to detect the leaching of sodium by the sample from ordinary glass.
The inflection points will be quite pronounced and easy to see. For this study, a DG116 nonaqueous pH electrode (METTLER TOLEDO) was used. Relative standard deviations of 0.2% on blank samples were obtained, and on samples that were 0.0045 mg KOH/g, RSDs of 5.0% were obtained, which is very promising considering the very low level of acidity. Testing was done on a 5-mL burette with equivalence points routinely realized at 0.3 mL, which is well outside the optimum range of the 5-mL burette. A 95% confidence interval of 1.0 nEq/g is possible with the procedure described. The theory of this titration can be used in additional applications where a balance of acidity/basicity is needed.

Figure 4 - Excellence titrator.
This method can be run on the most basic METTLER TOLEDO Excellence titrator, the T50 (see Figure 4). Since this is a back titration, an additional dosing unit with a 20-mL burette is required for automated analyses. Greater flexibility can be gained by running this method with either the T70 or T90 units, which can handle a total of four or eight burette drives, respectively. This added degree of flexibility will allow for both the acid/base balance and molecular weight/hydroxyl number titrations to be on the same instrument since they are both back titrations and common analytical test methods required in polyol production.
An important requirement of optimizing this method is keeping the electrode clean.The polyol sample has an affinity to stick to the electrode, which results in decreasing electrode sensitivity over time. A cleaning procedure that includes a THF soaking is strongly recommended. This can be done manually; however, the authors noticed too much variability from operator to operator to recommend this as the most effective utilization of resources. The problem was solved by inserting two conditioning steps into the method and running each sample on the Rondo20 autosampler (METTLERTOLEDO). Upon completion of each sample, the autosampler would soak the electrode in a THF beaker for 30 sec with stirring to facilitate cleaning. Then it would allow the electrode to condition in a 50% isopropyl alcohol/50% deioinized water solution for 60 sec. This proved to be a very reliable means to ensure that the electrode was clean and hydrated at the end of each sample.
The following reagents were used:
- Methanol (EM-MX0475-4) (EMD Chemicals, Gibbstown, NJ)
- Isopropanol (EM-PX1835P-4) (EMD Chemicals)
- Tetrahydrofuran stabilized with BHT
- p-Toluenesulfonic acid monohydrate (EM-TX0770-3) (EMD Chemicals)
- Lithium chloride (EM-LX0330-2) (EMD Chemicals)
- Tris(hydroxymethyl) aminomethane (THAM) (EM-TX1529-1) (EMD Chemicals)
- Sodium bi-carbonate (EM-SX0320-1) (EMD Chemicals).
Mr. Hynes is Instrument Sales Specialist, Titration, Density, and Refractometry Products, METTLER TOLEDO, 1900 Polaris Pkwy., Columbus, OH 43240, U.S.A.; tel.: 800-METTLER; fax: 614-985-9310; e-mail: [email protected]. Dr. Koile is President, Koile & Associates, Inc., League City, TX, U.S.A.