Specifying a cell storage system is an important decision
for any life sciences laboratory. A poor choice can put
valuable research at risk and add costs. A good choice
can reduce aggravation and lower expenditures. Planners
need to consider a variety of factors when selecting a cell storage system, including temperature needs, power
demands, sample contamination, potential facility
requirements, safety, and cryogen supply. This article
compares the two types of storage systems—liquid nitrogen (LIN) freezers and mechanical freezers—to help the
reader make more informed purchases.
Temperature
The first question that a planner should ask when
selecting a storage system is “How cold is cold?” For
many applications, cold enough means achieving the
glass transition temperature of water (–130 °C). At
this temperature, movement within a cell ceases,
putting it into a state of suspended animation. This
enables the sample to survive for a virtually indefinite
period of time.
There are two primary options for storing samples at
this temperature: 1) LIN freezers and 2) mechanical
freezers. LIN units offer the coldest temperatures.
They store samples in a pool of LIN at –196 °C, or, in
the case of a vapor phase unit, in cold nitrogen vapor
at temperatures of –170 to –190 °C. Mechanical freezers
store samples at somewhat warmer temperatures.
The coldest mechanical units achieve temperatures of
–130 °C to –150 °C, although the vast majority of
mechanical freezers are designed to cool to –80 °C.
In general, mechanical units are the more convenient
option. The user simply plugs in the unit and
sets the temperature to get started. However, to
achieve temperatures at or below –130 °C, mechanical
units must operate at the very edge of their
ranges, making samples somewhat vulnerable to fluctuations
in temperature. Consequently, these units
are still somewhat rare. In contrast, LIN units are the
more common and more reliable option for storage
below the glass transition temperature and for long-term
sample preservation.
Sample integrity

Figure 1 - LABS 40K freezer.
In recent years, the potential for contaminants such
as viruses, bacteria, and fungi to migrate from cell to
cell when immersed in LIN has become an issue.
Laboratories are under pressure to protect samples
from contamination. One way for them to do this is
to provide additional protective containers around
the vials that contain the samples. Another way is to
store samples in a vapor phase unit, which keeps the
samples below the glass transition temperature, without
direct contact with the LIN.
The argument has been made that LIN vapor phase
units have large temperature gradients and therefore
provide inconsistent freezing. This may be true for
some older units that have large lids. However,
newer designs, equipped with improved control systems,
maintain extremely consistent temperatures.
For example, in a temperature profile test, the LABS 40K™ unit, equipped with a KRYOS® control system (Taylor-Wharton Cryogenics, Theodore, AL)
(Figure 1), demonstrated the ability to maintain a
consistent temperature of –180 to –187 °C across the
top of the racks. In fact, when the lid was left open
for 3 hr, the temperature still held within a range of
–171 to –181 °C (see Figure 2). It is unlikely that a
mechanical unit would perform as well with its door
open for this extended period of time.
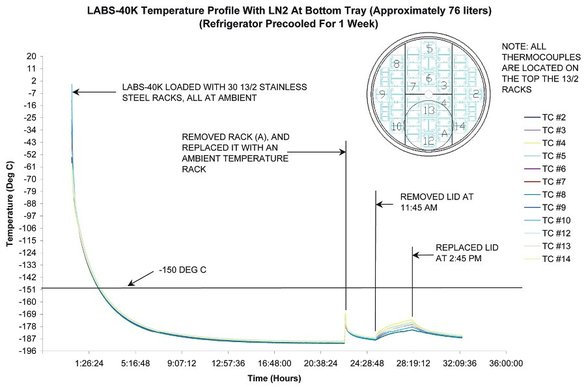
Figure 2 - Temperature of vapor phase unit.
Storage above the glass
transition temperature
Conventional wisdom says that because mechanical
freezers are convenient, they are the natural
choice for storage above the glass transition temperature.
However, the choice is not so simple.
Users need to consider a host of factors, some of
which may make an LIN freezer the practical
choice above the glass transition temperature also.
In reality, most facilities use a combination of
mechanical and LIN units to meet their needs.
Following are some factors to consider when making
decisions for specific applications.
Facility planning issues
Facility issues such as power demands, space, location,
cleanroom, and secure access requirements all
play important roles. Users should consider questions
such as, “How much electricity will I need to operate
the freezers?” and “How do I supply units on upper
floors?” A discussion of these issues follows.
Power
Power consumption is a primary consideration. All storage
systems require some electricity to operate. However,
mechanical freezers consume much more power to run
compressors and refrigeration systems that achieve low
temperatures. The electricity to run a mechanical freezer
costs approx. $1100–$2200 per year, depending on the
local price of electricity. Most facilities have 20–30 freezers;
thus the annual power consumption can easily cost
$44,000–$66,000. Conversely, an LIN unit consumes
less power than a light bulb to run its automated controls.
It achieves its low temperatures through cryogens.
Added to this higher power demand is air conditioning
the room with mechanical freezers. The compressors
on a mechanical unit emit about 3200 BTU of
heat per hour. A room could quickly go from 70 to 100
°F, putting an increased load on the air conditioning
system. For one –80 °C unit, this will add $300 per
year, depending on local power costs. For the entire
facility, this could mean an additional $6000–$9000
power cost. In some older facilities, sufficient air conditioning
may not be available, requiring upgrades
that may make mechanical freezers cost prohibitive.
Also, the possibility of a power disruption should be considered.
During a disaster, or even a more common
brownout or blackout, the lack of power can put samples
at risk, especially if the proper backup systems are not in
place. Mechanical freezers operate much in the same way
as household freezers—without power, the unit does not
generate cold temperatures. The typical –80 °C mechanical
unit therefore uses high-pressure CO2 cylinders that
keep the unit cold for approx. 5–7 hr if the power goes
down. One should keep in mind that the gas coming from
the cylinder is –50 °C, which is warmer than the intended
temperature of a –80 °C freezer. For colder temperatures,
an LIN backup cylinder is required. Conversely, a typical
LIN freezer deprived of electricity can keep samples below
the glass transition temperature for up to 10 days, provided
the lid is kept closed. Backup systems will be discussed in
detail below in the section on managing the supply chain.
Space
Because space is at a premium in most biotechnology laboratories,
planners need to carefully consider their short- and
long-term space needs when selecting units. A typical
–80 °C freezer, with a capacity of 48,000 vials, takes up a little
over 26 ft2, including the space to open the door and a
6-in. space around the unit for airflow. The unit may
require a 9-in.-diam CO2 cylinder for backup. In contrast,
a LABS 40K unit takes about 17 ft2 to hold 41,500 vials
(see Figure 3). It must be kept in mind that the LIN unit
uses a 26-in.-diam LIN cylinder. If a laboratory chooses to
keep the LIN cylinder right next to the unit, the space
requirement is about 30 ft2. However, planners may want
to consider implementing alternative supply modes such as
an external bulk or microbulk tanks located in less spaceconstrained
areas to conserve space in the freezer room.
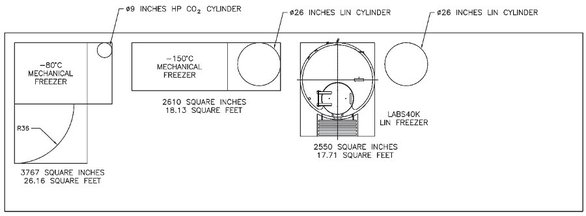
Figure
3 - Unit dimensions.
Another consideration is that LIN freezers are a good
option in places in which headspace is limited. LIN
freezers are built chest style—the average LIN unit is
a little less than 4.5 ft tall, significantly shorter than
a 6.5-ft mechanical freezer. An additional 1.5 ft of
headspace should be allowed for the lid to open so
that personnel can retrieve samples when necessary.
Weight and facility
limitations
When selecting units for upper floors, weight limitations
can restrict the size of the individual storage
unit. The evaluation can become very detailed and
specific; therefore it is a good idea to review the building
codes prior to selecting a unit. Additionally, the
physical dimensions of elevators,
doorways, and corridors may
limit the choice of units for upper
floors. For example, a larger unit
may be ideal for a first-floor
installation but may not work on
a second floor if there is not an
elevator of suitable size. Likewise,
larger units may not fit through
narrow doors or corridors.
Validation
Validation is a process that has to be undertaken for
both mechanical and LIN units. Validation includes
several processes, including installation qualification
(IQ), operational qualification (OQ), and performance
qualification (PQ)—costing approx. $1000.
This cost can increase depending on individual customer
specifications and needs to be factored into
the cost for each unit. In general, the fewer the units,
the lower the validation cost. Thus, where possible,
one should consider selecting the largest unit in a
series to minimize this cost. For example, a LABS
80K™ unit provides about the same storage capacity
of two LABS 40K units at half the validation cost.