For the past two decades, enzyme electrode development and applications have received a great deal of attention.1‒5 The conventional enzyme immobilization method depends on the preparation of an enzyme membrane attached to the electrode surface with a fixation device such as a holder, an O-ring6 and nylon mesh.7 However, less expensive, safer, more readily available and effective enzyme immobilization methods and materials are still being explored.
The sol-gel technique is widely used for the immobilization of biomolecules, in particular, enzymes.8,9 The porosity and network structure of sol-gel materials can provide a favorable microenvironment for enzyme immobilization. Moreover, their biocompatibility can preserve the catalytic activities of enzymes.10 Nevertheless, sol-gel materials still have obstacles, the main being their tendency to crack.11
This article describes a new approach to the fabrication of an acetylcholinesterase (AchE) electrode based on an improved sol-gel material. Immobilized AchE was successfully attached to the bulb surface of a pH glass electrode. It was found that the sol-gel membrane entrapping AchE avoided the problem of cracking, and the AchE-based biosensor can be applied to detect organophosphorus pesticides based on the inhibition effect.
Experimental
Immobilization of sol-gel-AchE film onto the surface of the pH electrode
Tetramethyl orthosilicate (13.0 mL) with 14.4 mL anhydrous ethanol were mixed in a 50-mL beaker. Hydrochloric acid (0.15 mL of 0.1 M) was added and the reaction mixture was stirred at 30 °C for 24 hours. Subsequently, the beaker was covered with paper and remained for 12 hours at room temperature without stirring. Sol-gel (4.0 mL) was infused into a 5-mL beaker and 0.3 mg solid AchE was added. The solid enzyme was dissolved in the sol-gel by stirring with a glass rod. After 3‒5 minutes, a uniform sol-gel-AchE liquid was obtained. Next, the pH glass electrode was kept in a vertical position and dipped into the sol-gel-AchE liquid for 2‒3 minutes. The electrode was withdrawn and left to dry for 4‒5 hours in a container of doubly deionized water. The electrode was held in a reverse vertical position with the water below it. The above procedures were repeated three times. In the last step, the drying of the sol-gel-AchE was extended to 30 hours.
Measurement procedure
The sol-gel-AchE immobilized pH electrode was immersed into a 10-mL KCl solution (1.0 mM) while stirring. The pH signal was captured by the pH meter and recorded using a personal computer. When the pH value became steady after a few seconds, a series of known standard acetylcholine chloride (Ach) solutions, the substance catalyzed by AchE, was injected with a microsyringe into the 10-mL KCl. The pH was continually monitored by the pH glass electrode.
AchE inhibition assay
The inhibition effect of malathion on the sol-gel-AchE biosensor was assessed as follows: The initial response of the biosensor to a 600 μg/mL Ach solution was taken. The biosensor was then cleaned and exposed to a known concentration of malathion with stirring for 25 min. After the inhibition reaction, the response was reassessed by exposing it to a fresh 600 μg/mL Ach solution. The inhibition effect was determined by the difference in the biosensor response to Ach before and after exposure to malathion.
Results and discussion
Surface morphology of the sol-gel film
Scanning electron microscopy (SEM) was used to determine the surface morphology of the sol-gel film coated on the pH electrode. Figure 1a and b show the SEM images of the sol-gel film on the electrode bulb surface coated once using the sol-gel process (see Experimental section) at a magnification of 3000 and 30,000, respectively. One sol-gel coating resulted in island-like structures that separated from each other. Figure 1a depicts the SEM image of the sol-gel-modified electrode with one coating; Figure 1b shows the physical morphology of one of the sol-gel particles.
 Figure 1 ‒ Scanning electron micrographs of the pH glass electrode bulb surface immobilized with the sol-gel film applied one time at 3000 magnification (a) and 30,000 magnification (b); the sol-gel film applied three times at 3000 magnification (c) and 30,000 magnification (d); sol-gel film applied four times (e).
Figure 1 ‒ Scanning electron micrographs of the pH glass electrode bulb surface immobilized with the sol-gel film applied one time at 3000 magnification (a) and 30,000 magnification (b); the sol-gel film applied three times at 3000 magnification (c) and 30,000 magnification (d); sol-gel film applied four times (e).The SEM micrographs display the sol-gel film on the electrode bulb surface coated by the sol-gel process three times at a magnification of 3000 and 30,000 in Figure 1c and d, respectively. After the third coating of the sol-gel, the isolated islands became more dense and smaller than in Figure 1a. The compactness, uniformity, porosity and network structure provided significantly enhanced effective electrode surface for high enzyme loading and a favorable microenvironment for the immobilization. In addition, the performance of the biosensor was improved considerably.
Figure 1e shows the SEM image of the electrode bulb surface coated four times by the sol-gel process. The film was so thick that it cracked. The chapped, coarse, thick film greatly hinders the biosensor’s capability, i.e., long response time and low sensitivity. Hence, it was not appropriate to employ the sol-gel coating four or more times; three times was deemed most effective.
Response of AchE biosensor
Eq. (1) illustrates the response mechanism of the sol-gel-AchE biosensor to Ach. The enzyme AchE catalytically hydrolyzes Ach to acetic acid and choline, accompanied by a decrease in pH (ΔpH).

The response to the Ach curve of the biosensor toward both the log values of various concentrations of Ach solutions (log [Ach]) and response time is presented in Figure 2. From 4 μg/mL to 720 μg/mL, ΔpH against log [Ach] is linear correlation. The curve displays ΔpH equal to 0.9939 log [Ach] minus 0.2905; the correlation coefficient is r = 0.9929.
 Figure 2 ‒ Response curves of the sol-gel-AchE biosensor after each successive addition of various concentrations of AchE: (1) 0, (2) 1, (3) 4, (4) 10, (5) 20, (6) 35, (7) 55, (8) 80, (9) 110, (10) 150, (11) 200, (12) 260, (13) 330, (14) 410, (15) 500, (16) 600, (17) 720 μg/mL AchE.
Figure 2 ‒ Response curves of the sol-gel-AchE biosensor after each successive addition of various concentrations of AchE: (1) 0, (2) 1, (3) 4, (4) 10, (5) 20, (6) 35, (7) 55, (8) 80, (9) 110, (10) 150, (11) 200, (12) 260, (13) 330, (14) 410, (15) 500, (16) 600, (17) 720 μg/mL AchE.Effect of temperature
As shown in Figure 3, the temperature influences the response rate of the biosensor. The response rate increased with the increase in temperature in the range 10‒30 °C. When the temperature was above 30 °C, the response rate decreased gradually with the increase in temperature. A possible reason is that the immobilized AchE may acquire higher activities at higher temperatures (10‒30 °C) with the subsequent effect on producing faster signal change during the catalytic hydrolysis of Ach by AchE. However, when the temperature is too high, the enzyme is possibly denatured, resulting in a decrease in the response rate of the biosensor. Although the response rate is highest at 30 °C, room temperature (25 °C) was chosen in this work for practicality and convenience.
 Figure 3 ‒ Effect of temperature on biosensor responses.
Figure 3 ‒ Effect of temperature on biosensor responses.Shelf-life of the sol-gel-AchE biosensor
Figure 4 depicts the shelf-life of the sol-gel-AchE biosensor. The sensitivity increased 15% of its initial value during the first five days of storage. This may be due to the increase in the permeability of the sol-gel film to Ach during this film-swelling period.12,13 After this, the sensitivity decreased slowly the longer the biosensor was stored. Fortunately, the biosensor still maintained 100% of its original sensitivity (after approximately 210 measurements) on the 20th day. In other words, the sol-gel entrapped enzyme was well protected using immobilization method, without a loss in activity, after 210 measurements.
 Figure 4 ‒ Shelf-life of sol-gel-AchE biosensor.
Figure 4 ‒ Shelf-life of sol-gel-AchE biosensor.After 30 days, the sensitivity of the biosensor decreased more significantly. Finally, it was able to retain 61% of its initial activity after 80 days of storage. The decrease in sensitivity may be attributed to the gradual denaturation of the immobilized AchE and/or enzyme leakage after a prolong usage.
Determination of malathion
It is well-known that pesticides can inhibit the activity of AchE. In this work, the response of the sol-gel-AchE biosensor Ach was initially determined and then exposed to a known concentration of malathion for 25 minutes. The biosensor was rinsed with deionized water and its response to Ach was reassessed. The inhibition effect (I%) of malathion is calculated as follows:
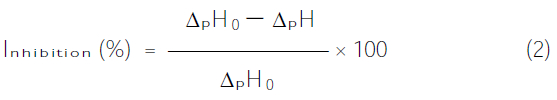
Where ΔpHo and ΔpH are the response of the biosensor to Ach before and after exposure to a known concentration of malathion, respectively. Three replicates were done for each measurement. An incubation time of 25 minutes was selected as the best compromise between signal and exposure time.
Figure 5 shows the inhibition effect of malathion on the biosensor by plotting I against ‒log [malathion]. The inhibition of the immobilized AchE activity increased with the concentration of malathion ranging from 10‒11 to 10‒6. The relationship is linear in the range 5 × 10‒11‒10‒7: I (%) = 12.18 log [malathion] + 132.1 with r = 0.9910. If I is kept at 10%,14‒16 the detection limit for malathion is determined to be 0.2978 nM. The RSD for determining malathion in the range 5 × 10‒11‒10‒7 is less than 8.0% (n = 5). The recovery percentages ranged from 90.5 % to 110.0 % for the determination of malathion in domestic wastewater treated at municipal sewage treatment plants. The biosensor is thus suitable for real-world applications.
 Figure 5 ‒ Inhibition effect of malathion on the sol-gel-AchE biosensor. Plot of I against log [malathion].
Figure 5 ‒ Inhibition effect of malathion on the sol-gel-AchE biosensor. Plot of I against log [malathion].Conclusion
A new sol-gel-AchE film can be directly attached to the bulb surface of a pH glass electrode for AchE biosensing. The sol-gel film as prepared can prevent cracking, a common occurrence in enzyme-entrapped sol-gel film. The sol-gel-AchE biosensor has been used successfully to determine Ach and malathion. In addition, the biosensor fabrication method is simple, convenient and nontoxic.
References
- Sampath, S. and Lev, O. Inert metal-modified, composite ceramic-carbon, amperometricbiosensors: renewable, controlled reactive layer. Anal. Chem. 1996, 68, 2015‒21.
- Shin, J.H.; Marxer, S.M.et al. Nitric oxide-releasing sol-gel particle/polyurethane glucose biosensors. Anal. Chem. 2004, 76, 4543‒9.
- Ges, I.A. and Baudenbacher, F. Enzyme electrodes to monitor glucose consumption of single cardiac myocytes in sub-nanoliter volumes. Biosens. Bioelectron. 2010, 25, 1019‒24.
- Ghica, M.E.; Carvalho, R.C. et al. Glucose oxidase enzyme inhibition sensors for heavy metals at carbon film electrodes modified with cobalt or copper hexacyanoferrate. Sens. Actuators B 2013, 178, 270‒8.
- Arken, G.L.J.; Li G.X. et al. A novel electrochemically deposited hybrid film for an electrogenerated chemiluminescence sensor. Anal. Lett.2014, 47, 2522‒36.
- Xiao, D. and Choi, M.M.F. Aspartame optical biosensor with bienzyme-immobilized eggshell membrane and oxygen-sensitive optode membrane. Anal. Chem. 2002, 74, 863‒70.
- ConclusionAbad, J.M.; Pariente, F.et al. Determination of organophosphorus and carbamate pesticides using a piezoelectric biosensor. Anal. Chem.1998, 70, 2848‒55.
- Rosatto, S.S.; Sotomayor, P.T. et al. SiO2/Nb2O5 sol-gel as a support for HRP immobilization in biosensor preparation for phenol detection. Electrochim. Acta 2002, 47, 4451‒8.
- Yashina, E.I.; Borisova, A.V. et al. Sol-gel immobilization of lactate oxidase from organic solvent: toward the advanced lactate biosensor. Anal. Chem.2010, 82, 1601‒4.
- Pandey, P.C.; Upadhyay S. et al. A new solid-state pH sensor and its application in the construction of all solid-state urea biosensor. Electroanalysis 2000, 12, 517‒21.
- Lev, O.; Tsionsky, M. et al. Organically modified sol-gel sensors. Anal. Chem. 1995, 67, 22A‒30A.
- Yang, Q.; Atanasov, P. et al. Enzyme electrodes with glucose oxidase immobilized on stober glass beads. Anal. Lett. 1995, 28, 2439‒57.
- Mitchell, K.M. Acetylcholine and choline amperometric enzyme sensors characterized in vitro and in vivo. Anal. Chem. 2004, 76, 1098‒1106.
- Jin, S.Y.; Xu, Z.C. et al. Determination of organophosphate and carbamate pesticides based on enzyme inhibition using a pH-sensitive fluorescence probe. Anal. Chim.Acta 2004, 523, 117‒23.
- Nunes, G.S.; Jeanty, G. Enzyme immobilization procedures on screen-printed electrodes used for the detection of anticholinesterase pesticides comparative study. Anal. Chim.Acta 2004, 523, 107‒115.
- Jeanty G. and Marty, J.L. Detection of paraoxon by continuous flow system based enzyme sensor. Biosens. Bioelectron. 1998, 13, 213‒18.
Prof. Dan Xiao, corresponding author, is with the College of Chemistry, Sichuan University, Chengdu 610064, PR China; tel.: +86 28 85415029; fax: +86 28 85416029; e-mail: [email protected]. Jie He is also with the College of Chemistry, Sichuan University, and with the Monitoring Center, Chengdu Drainage Co., Ltd., Chengdu, PR China. Hongyan Yuan is with the College of Chemical Engineering, Sichuan University, Chengdu, PR China. Runguo Lin is with the Guangxi Research Institute of Chemical Industry, Nanning, PR China. Martin M.F. Choi is partner, Department of Chemistry, Hong Kong Baptist University, Kowloon Tong, Hong Kong SAR, PR China. Financial support from the National Natural Science Foundation of China (grant nos. 21177090, 21275104 and 21175094) and the Guangxi Science Foundation of China (no. 0229012) is gratefully acknowledged.