When the Human Genome Project mapped all 20,000+ genes in the human body in the late 1990s and early 2000s, it cost roughly $3 billion. Today, advances in next-generation sequencing technology allow an individual’s genome to be sequenced for closer to $1000 and in just three hours. But sequencing a person’s genome is only the first step in delivering personalized medicine. Molecular diagnostic techniques provide more than just the patient’s genetic information; they help us to understand a person’s unique physiology and how they will respond to a particular treatment. While access to this information has enabled doctors to fundamentally alter their approach to diagnosing and treating diseases such as cancer, it also brings new challenges. Chief among these challenges is managing data—vast amounts of data—and this has required a paradigm shift in the medical industry as a whole.
In the hospital or clinic setting, labs have traditionally delivered their test results through the laboratory information system (LIS). The LIS is well suited to routine testing and delivery of results to physicians and, because it was also designed for managing patient care and billing, the LIS has also been designed to maintain patients’ privacy in compliance with the Health Insurance Portability and Accountability Act (HIPAA). The LIS, however, was not designed to manage the large sample volume, elaborate laboratory process, or complexity of the results generated by molecular diagnostic testing.
In contrast to the hospital environment, life science research and development organizations have been performing genomic, proteomic, and metabolomic testing for over a decade to help understand which drugs are safe and efficacious enough to undergo clinical trials and ultimately be approved by the FDA and other regulatory agencies around the world for human use. These laboratories have relied on the laboratory information management system (LIMS), which is optimized for next-generation sequencing and other analytical testing. Patient information was kept out of the LIMS in these laboratories to prevent scientific bias, so HIPAA compliance was never a concern.
As the fields of translational and personalized medicine have evolved, a connection has been made between the laboratory bench and patient care. This emphasis on bench-to-bedside medicine brought new information management challenges. Today’s patient care relies on these sophisticated tests, and as a result there has been a call for a convergence of these systems, which up to now have been separate. Physicians are now able to improve patient diagnosis and treatment because they have a better understanding of the unique genetic makeup of patients and their diseases, and rely on dedicated molecular diagnostic laboratories that offer specialized testing for their patients’ illnesses. To support this new paradigm, there needed to be a convergence of the lab-centric functionality found in a LIMS—which is designed to facilitate lab information, sample tracking, lab work flow, and results—with the functionality traditionally found in an LIS—which manages administration, billing, and secure patient information.
As personalized medicine has evolved, so too has the system of record to support its massive data requirements. Clinical laboratory information management systems (Clinical LIMS) combine the patient management capabilities of LIS with the lab process-focused approach and data capacity of LIMS, which are used throughout life science research laboratories. The new, more powerful Clinical LIMS enable physicians to apply valuable genetic data to patient care, paving the way for diagnosis and treatment that is truly personalized. The convergent technology found in a Clinical LIMS is unifying the distinct functionality required of physicians in hospital settings, as well as that required by researchers in clinical testing labs.
Why personalized medicine?
According to the American Cancer Society, early detection of cancer has been proven to “reduce mortality from cancers of the colon and rectum, breast, uterine cervix, and lung.” In breast cancer, for example, if a patient is diagnosed while the cancer is at a localized stage, the five-year survival rate is 98%. That number drops to 15% if the disease is diagnosed at stage IV.
Earlier diagnosis leads to better outcomes for patients—that much is clear. But with an aging U.S. population putting increased pressure on the health-care system, better diagnosis and treatment of costly diseases such as cancer is critical. Improved analytical instrumentation and research developments have done much to advance medicine, but there is still much work to be done at the patient level—on a personalized basis.
The potential for personalized medicine goes far beyond cancer alone. Markers for heart disease, diabetes, and Alzheimer’s, for example, are also discoverable in a person’s genetic code. Early diagnosis of these conditions is crucial, because it enables patients to make lifestyle changes or undergo treatments that can significantly prolong their lives and reduce the burden on the health-care system as a whole.
Drug discovery and development
No discussion of personalized medicine is complete without considering advancements in drug discovery and development. The pharmaceutical industry has made unprecedented progress in developing novel drugs and methods to identify which members of the larger population would benefit from new therapies. These advancements have given rise to companion diagnostics, which, although not the whole solution, are enabling clinicians and physicians to build treatment plans that are far more personalized to each patient and cost-effective for the system overall. In advance of prescribing a treatment, physicians can now use biomarkers to go beyond symptoms and prove at a genetic level that a particular drug may work for an individual patient.
But again, a doctor’s ability to personalize treatment is only as good as his or her ability to access well-managed data. The wealth of genetic information cannot improve care without a system to track samples, collate information, and store data for later retrieval.
The convergence of LIMS and LIS
Today, personalized medicine creates demand for a system that combines features of both a LIMS and an LIS. Clinical laboratories require an end-to-end system that manages all information related to patients, from point-of-care testing and diagnostics to treatment. This often includes execution of complex, sample-oriented work flows (including data from sophisticated, automated instruments) while ensuring good laboratory practices, regulatory compliance, and the security of patient information. A true Clinical LIMS meets these requirements by delivering the best of both worlds—a convergence of functionality between a traditional LIS, which manages patient care, and a LIMS, which manages dynamic work flows across a lab, including everything from sample tracking to results reporting.
A Clinical LIMS does not simply improve the data management capabilities of an LIS—it does so much more by bringing in a fundamental understanding of lab processes. This is clear with traceability, for example, which was not a traditional focus for LIS but is now mission critical in personalized medicine sample work flows, where volumes are so much greater. Because Clinical LIMS evolved from high-throughput, highly regulated environments, they are more than capable of tracing samples as they travel through various testing and storage checkpoints.
Clinical LIMS in practice
The combination of LIMS and LIS may sound like an excellent idea, but how does it work in practice? Foundation Medicine, a Cambridge, MA-based cancer diagnostics company at the forefront of bringing comprehensive genomic analysis to routine cancer care, is already successfully using a Clinical LIMS as a personalized medicine enabler. FoundationOne, a clinical diagnostic test developed by Foundation Medicine, performs next-generation sequencing on a very small amount of genomic material. The test is used to develop a “molecular” blueprint of a patient’s tumor; the blueprint is then shared with the patient’s physician, who in turn uses it to outline relevant therapies, treatments, and clinical trials.
Developing a molecular blueprint suitable for diagnostics and treatment requires a highly scaleable information technology infrastructure built for this specific purpose. While research labs are complex environments, a clinical setting adds another set of onerous requirements, from logical, patient-centered ways to order tests to administrative and business rules common in health-care organizations and regulations concerning data security and patient privacy. Further complexity comes from electronic medical records (EMRs), which not only contain all information associated with a patient, from a doctor’s notes about a medical visit and medication history to medical test results, but also serve as the physician’s main interface into the LIS.
With a presence in both the clinical and laboratory environments, Foundation Medicine required the best of both worlds, and a Clinical LIMS was the answer. A Clinical LIMS also ensured that patient data would be managed securely and with the appropriate privacy settings to keep a facility in compliance with HIPAA and CLIA (Clinical Laboratory Improvement Amendments) regulations.
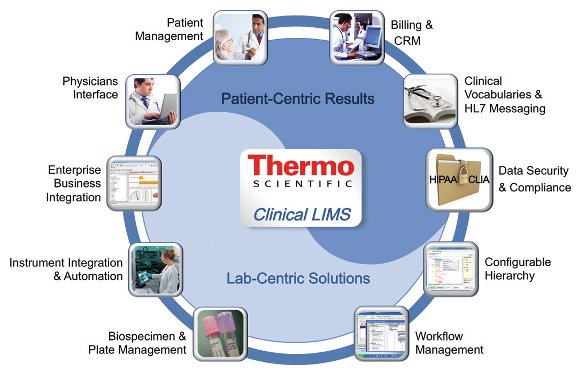 Figure 1 – A comprehensive solution that combines the sample-centric functionality of a LIMS with the patient-centric functionality inherent in a traditional LIS, Thermo Scientific Clinical LIMS bridges the gap between the two and delivers a complete and streamlined solution.
Figure 1 – A comprehensive solution that combines the sample-centric functionality of a LIMS with the patient-centric functionality inherent in a traditional LIS, Thermo Scientific Clinical LIMS bridges the gap between the two and delivers a complete and streamlined solution.Foundation Medicine chose the Thermo Fisher Scientific (Philadelphia, PA) Clinical LIMS solution (see Figure 1). It not only meets Foundation Medicine’s sample- and patient-centric requirements today, but is also able to evolve as the organization’s needs change. As personalized medicine advances, so too will Foundation Medicine’s LIMS.
 Figure 2 – The HL7 communication between the Clinical LIMS and external collaborators is facilitated by Thermo Scientific Integration Manager, allowing a streamlined end-to-end information flow following the patient from the point of care to molecular testing and results analysis, to diagnosis and treatment.
Figure 2 – The HL7 communication between the Clinical LIMS and external collaborators is facilitated by Thermo Scientific Integration Manager, allowing a streamlined end-to-end information flow following the patient from the point of care to molecular testing and results analysis, to diagnosis and treatment.Another benefit of the Clinical LIMS is how it assists Foundation Medicine with state and national inspections designed to ensure the lab is correctly processing clinical samples. These inspections include a random selection of patient samples and a review of the entire paper trail, from requisition forms to training documentation (for anyone who handles the sample), maintenance documentation (for all equipment that touches the samples), reagent logs, control verification logs, and quality control documentation (see Figure 2). Such detailed traceability requirements would be difficult to meet with an LIS.
Conclusion
Physicians and researchers throughout clinical, research, and academic laboratories are helping usher in a new era in patient care: the age of personalized medicine. They are tapping into a wealth of information, including electronic records and genetic information, and placing it as close to the point of care as possible. Without new systems to collect, manage, and interpret this information, all of these “big data” would have limited value.
Clinical LIMS may have their roots in research, far away from patients and their doctors, but the technology has evolved to meet this important need. Combining the strengths of a traditional LIS with a LIMS brings us one step closer to true personalized medicine. Although we are still in the early stages of personalized medicine, Clinical LIMS will play an important role moving forward, providing the health-care community with a sample-centric solution that, at its core, is also designed to help it deliver patient-centric results.
Trish Meek is Director of Product Strategy, Life Sciences, Thermo Fisher Scientific, 1601 Cherry St., Philadelphia, PA 19102, U.S.A.; tel.: 215-964-6020; e-mail: [email protected] ; www.thermofisher.com.